Table of Contents
If the laboratory is so located that coal gas or natural gas is accessible, the problem of heating hot plates and making ignitions simply resolves itself into the use of gas stoves and burners of the simplest types, such as are familiar to every student of chemistry. When, however, the laboratory is located, as most small ones are, at some mine, smelter or furnace, no such convenient means is at hand and something must be substituted for city gas. Where fire assays are made, the chemist will of course find the muffle of the assay furnace all that is needed for burning off his filter papers and igniting his precipitates; and oil stoves or stoves heated by wood or coal may be used to evaporate solutions, boil water, etc. In the east, in the laboratories of blast furnaces, cement mills and various and sundry manufactories, there are of course, no fire assays made and the heating here is usually done with gasolene.
This is used in one or two ways; either the gasolene is burned directly in a suitable burner or lamp, or else it is vaporized by a current of air and the mixture is burned in some form of Bunsen burner just as if it was coal gas. The latter method is the most convenient one but requires the use of a generator to vaporize the gasolene. The latter is somewhat expensive if purchased, but in many cases may be constructed by the mechanics employed at most manufacturing plants at a comparatively small cost. The lamps for burning gasolene directly are troublesome and not altogether free from danger, unless carefully handled. They require frequent attention, and usually have to be filled at least once a day.
Gasolene Lamps
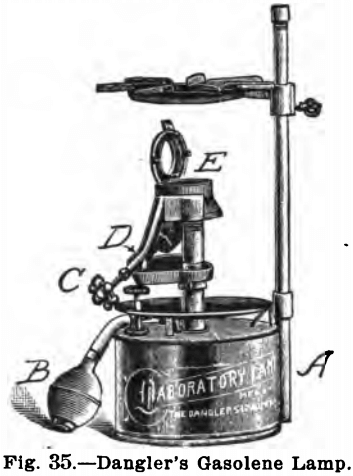
Of these lamps the best known is that of Dangler, which is shown in Fig. 35. In order to start the lamp the reservoir, A, is filled with gasolene, the bulb, B, is compressed a few times to put the gasolene under pressure and the valve, C, is opened enough to let a little gasolene into the pan D; this is then ignited with a match and when the burner has been heated, the gasolene is turned on, vaporized by the hot burner and ignited at E. These lamps give a flame which can be regulated from the size of that of an ordinary Bunsen burner to a powerful blast. They cost between $5.00 and $6.00, can be purchased from any supply house, and are very similar to the torches used by painters, plumbers, etc.
Figure 36 shows the burner manufactured by The Hoskins
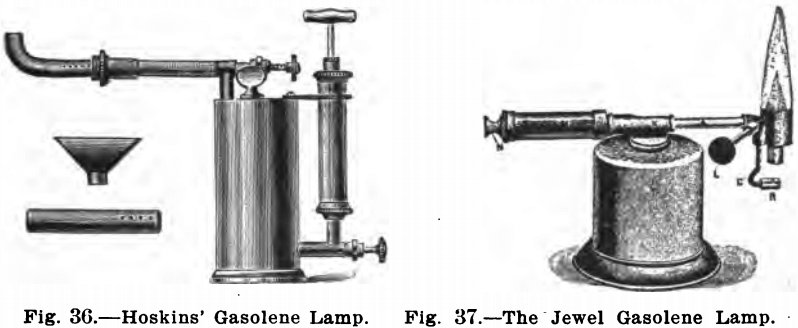
Company, 93 Erie St., Chicago. This burner is an improvement on the Dangler burner. It is more portable and the substitution of the metal pump for the rubber bulb, which wears out rapidly, is a good feature. The flame is under perfect control and the lamp is substantially put up. The extra tubes are for a fish tail flame, for bending glass, and one horizontally directed.
Figure 37 shows the Jewel gasolene lamp for sale by E. H. Sargent & Co., Chicago. This is a very small, new type of gasolene burner which meets all the purposes of a Bunsen burner. It generates its own gas and is practically automatic in operation. The tank or reservoir which forms the main body of the lamp holds about one-half pint of gasolene. Air pressure for operating this torch is obtained by means of a small force pump contained in the handle. The flame is adjustable in size from almost nothing to 5 to 6 inches, and will burn about 1¾ hours at full blast from one filling of the reservoir. The ease with which this burner is lighted is a most desirable feature. The heat of one or two matches is all that is necessary to generate the burner. Figure 38 shows the generator being heated preparatory to lighting the burner.
Stoves
For evaporating solutions, etc., a gasolene stove such as is used in kitchens will be found a necessary adjunct to the gasolene lamps. Or a kerosene stove may be used. These
latter can be obtained either with or without wicks, the wickless “blue flame” ones being the best, although a little more expensive than the older form. These stoves are usually mounted on an iron frame and are intended to stand directly on the floor. It will be found most convenient, however, to raise the stove six or more inches and to build a hood over it. The stove should be covered with a cast iron plate and, if many samples have to be dried or moisture determinations made, the ovens sold with these stoves for baking purposes may be used.
In place of the oil or gasolene stove, an ordinary wood or coal cooking stove may be used, or a hot plate may be rigged up over a grate, so as to be heated by wood or coal. Neither of these schemes are desirable, however, except where kerosene and gasolene can not be obtained. Mr. Herbert Haas describes such an arrangement designed by him for the laboratory of a pyrite smelter in California, as follows:
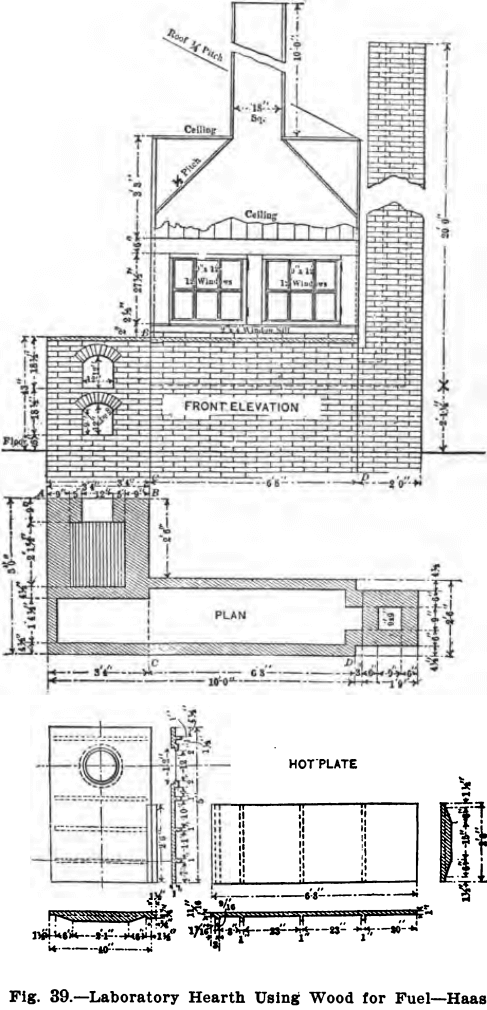
“The hearth, Fig. 39, rests on the ground and the flue from it is filled with ashes and earth to within 18 inches of the hot-plate. In front of the ash-pit, beneath floor, is a 12 x inch inch opening, which is closed with a piece of sheet iron luted on with clay. The accumulated ashes in the ash-pit are removed through this opening, thus avoiding their removal through the office. The ash-pit door, shown in the drawing, is used solely to regulate the draft. Old rails, preferably, are used as grate bars.
“The front elevation of the hearth is shown in the illustration, which includes also the elevation of the fireplace front, with the ash-pit door and the feed door. The lines AB and CD, explain the respective elevations. The walls of the hearth consist of one course of brick, excepting at the stack, which is of a course and a half, and the fire-box, which is of two courses. These walls support the hot-plate, having its upper surface 43 inches above the level of the floor. A detailed dimensioned drawing of the hot-plate is given in the lower part of the drawing. The plate is cast in two pieces, having a lap so that a tight joint may be obtained, and at given intervals ribs are cast as a safeguard against warping. A circular hole, over which the still is placed, is left in the plate. A portion of the hot-plate 2.5 feet by 6 feet 8 inches, is covered with a hood, which rests on one layer of bricks, except at the hottest parts, where there are two layers in order to protect the wood. The back side of the hood does not rest on bricks, but is separated from the plate by a 2-inch air space extending the entire length of 6 feet 8 inches. Access to the hot-plate is obtained through two windows, each having twelve lights of glass and hinge on the butts. The hood is tightly ceiled with tongue and groove lumber, and has an 18 x 18 inch wooden chimney, 10 feet high, to carry off the fumes. The temperature of the inside of the hood and hood chimney is sufficient to draw in fresh air constantly and thus improve the ventilation of the laboratory.
“The great advantage of the hot-plate is in its gradual decrease in temperature towards the chimney. The heating of solutions is generally started at the coolest place, and gradually continued toward the hottest part. The heat is diffused over a large area, and is not concentrated at one small spot, as is the case with a Bunsen burner; and the boiling over of solution is thus easily avoided at the expenditure of the least attention and care; this allows the chemist time in which to attend to other work. A small, uncovered portion, 2.5 feet by 3 feet 4 inches, is reserved for operations which are preferably conducted in the open air.
“A still, placed over the circular hole described above, provides the laboratory with about 14 gallons of distilled water daily. The still now used is called the Cuprigraph Sanitary Still, No. 11, and when once regulated requires very little additional attention. The hearth thus serves two purposes, one to provide the laboratory with distilled water, and the other to give the chemist, at the same time, a very efficient way of heating solutions. The distillation of the water utilizes much of the heat, yet the hearth is necessarily wasteful, the flue being too short for economizing fuel. The weekly fuel consumption, however, is only from one-half to
three-quarters of a cord of wood, which at $4.00 a cord is equivalent to an operating expense of from 28 to 42 cents a day.”
Gas Machines
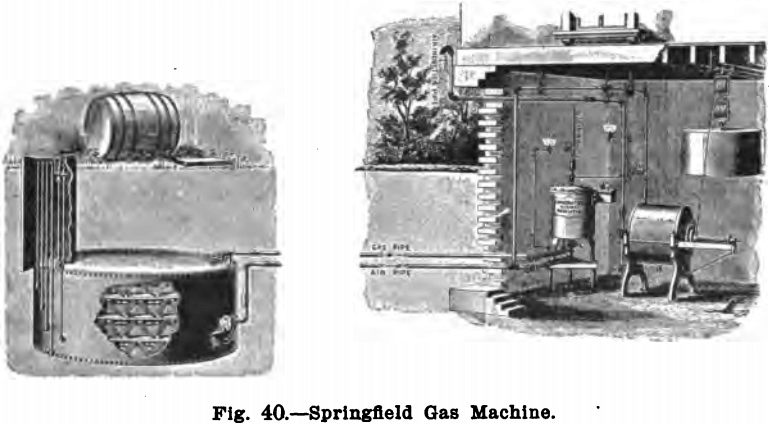
Of the machines for generating “gasolene gas” the “Springfield” is perhaps the best known and is manufactured by The Gilbert & Barker Manufacturing Co., 80 and 82 Fourth Ave., New York. The Springfield gas machine consists of a gas generator, which consists of a cylinder containing evaporating pans or chambers, a regulator for mixing the air and vapor in the proper proportions, and an automatic air-forcing apparatus.
The general plan of setting the apparatus, and the arrangement of connecting pipes, is shown in Fig. 40, which illustrates a Springfield gas machine, set up and connected, in every particular, in accordance with the latest rules of the New York Board of Fire Underwriters. The automatic air pump is here seen in the cellar of the laboratory, and connected to it and running in the ground is the air-pipe conveying air from this instrument to the gas generator, located underground, and removed from the building thirty, fifty, one hundred feet, or more.
When the machine is in operation, the pump forces a current of air through the generators; here it becomes carburetted, thus forming a combustible gas that is returned to the cellar, where it passes through the regulator and, if too rich in gasolene, air is added to make the mixture about 15 per cent, gasolene vapor and 85 per cent. air. From the mixer the gas goes to the burners.
Gas is generated only as fast and in such quantities as is required for immediate consumption. The process is continuous while the burners are in use, but instantly stops when the lights are extinguished. One gallon of gasolene will make about one thousand cubic feet of gas.
In place of the air pump operated by a weight, one run by water can be substituted. This latter form is to be preferred, as it does not need any attention. It does not require any head of water to operate, and two gallons of water are said to be sufficient to run one burner one hour. Both forms of pump give a steady pressure. These gas machines give excellent service and very little trouble.
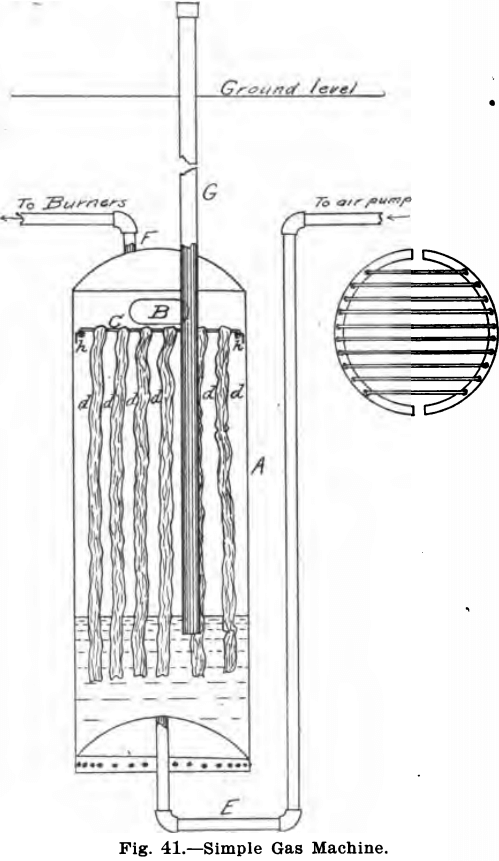
A machine which gives good results but which is not quite so convenient is described below. It is similar in the main to one designed for the laboratory of the Edison Portland Cement Co. by the author. The generator is shown in Fig. 41. It consists of an ordinary galvanized iron tank, such as is used in connection with kitchen ranges for holding hot water. A hole large enough to admit the hand is cut in the side of this tank at B, and two semi-circular pieces of light angle iron, bent to conform to the inside of the generator, are bolted inside as shown at h. The upper side of these angle irons are punched with holes, as illustrated in the small sectional drawing, and in these stout wires are fastened to make a sort of grid across the tank. Pieces of lamp wick, d, d, d, etc., long enough to reach to the bottom of the tank are hung down over these iron wires and the hole B is then covered by bolting on it a piece of metal. The joints of this, and also of all piping, are covered with solder, so as to prevent any possibility of a leak. The air pipe, E, and the gas pipe, F, and the fill pipe, G, should all have been screwed in place before hanging the wick over the wires. The tank is now buried in the ground. The pipe, F, should of course run to the laboratory and connect with the gas jets. The fill pipe, G, should reach 8 or 10 inches above the ground and be closed by a cap containing a washer. The pipe, E, leads to the air pump to be described later. The generator is filled with gasolene to a height of about 18 inches from the bottom, and fresh gasolene added every day or so to make up for that used. The height to which the gasolene has risen in the generator may be ascertained by fastening a small test tube on to a piece of stout iron wire, and noticing at what point this comes up full, when lowered into the tank, through the fill pipe, G. (In putting in the wires care should be exercised to allow room between the wires and wicking, at this point, to lower the tester.)
For furnishing the air for this generator a number of devices may be used. If only three or four burners are used at one time and a head of water is at hand nothing will be found so convenient as the water blower described in Chapter V. If more than this number of burners are to be used and plenty of water is at hand two or more of these blowers may be used, or a larger pipe or iron tank may be substituted for the pipe, B (Fig. 22), a larger over-flow pipe used and several aspirators screwed into this tank. One or more of these aspirators may be used as the amount of work done in the laboratory requires it.
In place of the water blower, if power is at hand, as is usually the case in most mill or furnace laboratories, the air may be supplied by a small Root or Crowell positive pressure blower, belted on to a shaft, in some convenient place about the mill. These blowers can be obtained from most dealers in laboratory supplies. Any means which will give a constant supply of air, at an unvarying pressure of about one pound per square inch, or even less, will answer the purpose of an air pump for this machine. The pressure of course must be low or the burners will be hard to light. Any change of pressure will also usually result in the flames dropping or being blown out.
This gas machine can be constructed with water blower for about $25 or with the Root or Crowell blower for about $40.00, depending very much on the amount of piping that has to be done.
Gasolene for these machines should be what is known as 88° and should be purchased if possible in iron drums. A spigot may then be screwed into the drum and the latter set on its side, or a suitable rest, and the gasolene drawn as required for replenishing the generator. The gasolene should be kept in a small shed, away from other buildings.
Acetylene
Acetylene gas is now used for laboratory purposes and quite a number of firms manufacture generators and burners especially suited to laboratory requirements. Acetylene gas gives off great heat when properly burned, but it requires special pattern Bunsens and stoves to burn it without loss. It is stated by one chemist who uses acetylene that it does not cause the deterioration of platinum ware which other forms of gas do. It compares very favorably with gasolene gas in economy.
Where much work is done with crucible furnaces and high temperatures are needed, acetylene gas may be the best gas to install, as it is possible with it to produce temperatures higher than those obtainable with gasolene gas or even with coal gas.
Burners
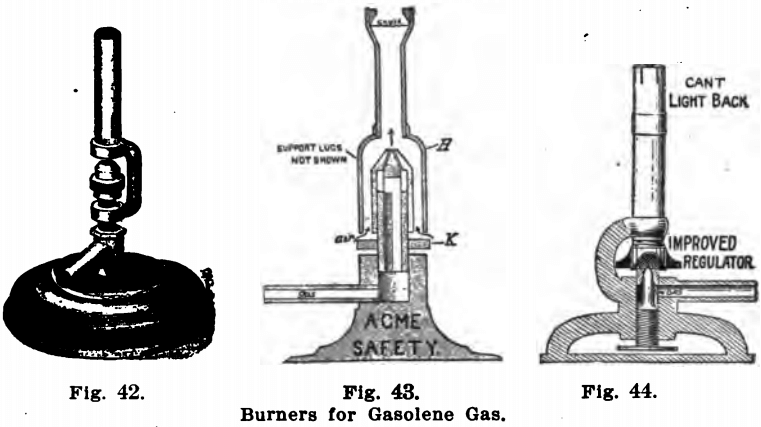
Figures 42, 43 and 44 show forms of burners well suited to gasolene gas. The author has always found the form shown in Fig. 42 to answer very well, but the other two burners have the additional advantage that they do not light back and hence are safer.
Where burners are to be used for heating dishes, a crown
or gauze top for the former distributes the heat more uniformly. For bending glass a wing top is needed. Stars, which slip over the tube of the burner and can be held at any height, are used to support chimneys of glass, sheet iron, or best mica. These chimneys keep the flame of the burner from being blown about by every draft. Julian has devised an adjustable support for dishes, platinum triangles, etc., which can be slipped over the tube of the burner and kept at any height by a thumbscrew. This does away with an independent support.
Porcelain burners are obtainable for use in hoods and other places where metal burners would be badly corroded. One of the neatest forms of these is Chaddock’s. As this burner is provided with a chimney, on which to set a triangle, and a support for dishes, etc., no metal tripod or retort stand has to be used.
When uniform heat is desired, as in evaporating solutions, an argand burner with chimney may be used. If ordinary burners are used, place on top the gauze on which the dish is supported, a round piece of asbestos paper the size of a silver dollar and then place the dish on top of this and the burner directly under it. This spreads the heat, and the evaporation can be made to proceed evenly without ebullition. Teclue burners are very powerful burners and are usually used for heating large sand, air or water-baths. Burners with from 2 to 6 tubes, either arranged in clusters or in a row, are also used for the same purpose.
Stoves and Hot Plates
The simplest hot plate is a sheet of boiler plate, 1/8 to ¼ inch thick, resting on a tripod and heated by a Bunsen burner. An improvement on this is a plate resting on a Fletcher’s burner. Since cast iron does not warp as bad as wrought iron a stove lid makes a good hot plate and may be used where only a little work is done, being heated by a Fletcher or a Teclue burner. Gas stoves may be procured either of chemical supply houses or else from local dealers in gas fixtures. The most convenient thing, however, is the regular hot plate sold by chemical supply houses and consisting of a gas stove having a polished cast steel top. The burners are arranged to give a uniform heat and they may be lit as needed so as to have one part of the plate hotter than the other. A hot plate 14½ x 18½ inches arranged for gasolene gas may be procured for $12.00.
Sand-baths are convenient for heating dishes and other round-bottomed utensils, but they are dirty things at best and unsuited to the analytical laboratory.
Water-Baths and Air-Baths
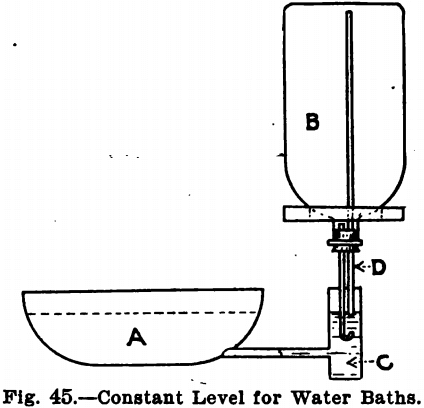
The water-bath is much used for evaporations and is made of both enameled ware and copper. They may be purchased with any number of holes, which are covered by concentric rings so that any sized dish may be set upon them. They may be heated by a Bunsen burner, or, if steam is run into the laboratory, they may be attached to this. Water-baths are also obtainable for keeping funnels and contents hot during filtering. Water-baths heated over a burner are liable from carelessness to boil dry. To guard against this, the constant level apparatus is used in some laboratories. This is shown in Fig. 45.
The bottle, B, is filled with water and closed with a single-hole cork having in it a piece of short glass tubing, d. This is placed in the cup, c, of the water-bath, A. When the water in A falls below the end of the tube, d, air enters the bottle and allows a corresponding amount of water to flow out. As soon as the water in the cup rises above the end of the tube, no more water, of course, can run out of the bottle. This is a clumsy arrangement, however, and may be dispensed with, as a very little experience teaches the chemist how much water he will require in his bath to run it the time he needs it.
Air baths for drying materials are needed in all laboratories, and are used for determining moisture in samples and for drying ores, coal, etc., etc. They usually consist of a copper box provided with a hinged door in front and holes for the insertion of a
thermometer and for the escape of water vapors in its top. The bath is provided with a false bottom, to prevent destruction of the real ore by the burner flame. The bath is heated by a Bunsen burner. It is necessary to control the temperature of the bath by a thermometer inserted through a cork in the opening at the top of the oven. The required temperature can be maintained by adjusting the stock cock of the gas supply. Gas regulators called “Thermostats” can be purchased from dealers in chemists’ supplies, and, while they are liable to get out of order by becoming clogged up, they are nevertheless very convenient for keeping a constant temperature. As any checking of the gas supply is likely to cause a gasolene gas burner to strike back, it is best, where a thermostat is used with this, to have two burners, only one of which is controlled by the thermostat. The other burner is turned low and the two burners are tilted so their tops are close together. If the burner attached to the thermostat goes out, as it probably will when the gas supply is checked, it will be lit by the other one.
Figure 46 shows a home-made thermostat. A piece of large bore tubing is blown into a small bulb, A, at one end and then about 2 inches from this bent into a U, B, as shown in the cut. This U is filled with mercury nearly up to the bulb. A piece
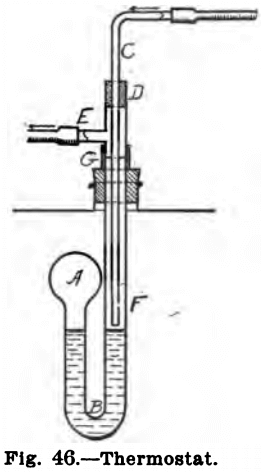
of thin tubing, C, having a small opening at F (not necessary if two burners are used) is inserted in the large tube and the opening between the two stopped by a piece of rubber tubing, D. The tube, C, is attached by rubber tubing to the burner and the tube, E, to the gas supply. The joint, at G, is to allow the apparatus to be inserted in the opening of the air-bath. To regulate, run the temperature up until the thermometer reads the maximum desired, then push the tube, C, down until it just goes below the surface of the mercury. This “Thermostat” is very delicate, but the rubber ring, D, sticks to the tubes and makes it hard to move the inner tube, C, up and down.
