Table of Contents
Man’s material wealth has usually started with fairly concentrated ore bodies that are mined, beneficiated, refined, and then manufactured into material objects. Following their use and abuse, the objects are discarded and wind up as spots of rust while the sources of raw materials become scattered haphazardly over the lithosphere.
Mining and metallurgical sorting practice has so far adopted classification, flotation, and magnetic separation as its principal tools. Because it is far easier to mix than to unmix, beneficiation is necessarily an energy-consuming step. This follows from the second law of thermodynamics and the principle of Planck/Boltzmann (S = klnW) , which relates probability, W (or disorder in the mixed state), to entropy, S, and conversely, relates information (or the ordered state) to negentropy (12)³ of “negative entropy.” Unable to redirect the distribution of minerals to a diminishing disorder or a negentropy state, and insisting on constantly chewing up the earth, man must develop new technologies to deal with marginal or lean deposits and to recover his needs from his wastes, especially so for his mineral needs.
Only a few minerals are commonly separated magnetically. Ordinary magnetic separation depends on the inherent magnetic susceptibility of the material to be separated. The distinction between magnetic and nonmagnetic minerals is quite arbitrary and depends on the types of magnets, field strength, and field gradients employed. In the iron ore industry, a magnetic fraction refers to iron oxides that are recoverable by a drum-type magnetic separator having a field intensity of 3 koe (2.39 x 10 5 amp-turn per meter) at its surface. This would attract magnetite (Fe3O4), which is technically ferrimagnetic, but not hematite (α-Fe2O3), although the latter compound is technically a weak ferromagnet with canted spin and can be attracted to higher field gradients as encountered in high-intensity magnetic separators. In this paper, the term “magnetic separation of the first kind” will be used to describe those separations in air or in water that rely on the magnetic susceptibility of the separated products.
The purpose of the present paper is to describe a new magnetic separation technique that does not rely on the inherent magnetic susceptibility, but rather on the specific gravity of the separated fractions. The technique will then be applied to segregate a typical waste product for materials recycle and to beneficiate a mineral to recover its precious content.
Magnetic Fluids
A magnetic fluid is a colloidal suspension of magnetic particles in a liquid carrier. The fluid remains in the liquid state even while being controlled, moved, or kinetically interacted with a magnetic field. Traditional methods of preparing magnetic fluids involve long-term tumbling of the magnetic material with steel balls for several weeks in a carrier medium containing the dispersing agent. In these grinding methods, oleic acid was commonly used to stabilize dispersions in kerosene and other hydrocarbon dispersion media. Small quantities of these fluids were thus prepared during the period 1966-71 at a cost of about $85 per 10 ml, or $32,000 per gallon. Research at the Bureau of Mines resulted in the development of chemical peptization methods to prepare magnetic fluids from magnetite in kerosene base in very short times and at the substantially lower cost of $3 to $4 per gallon or about $1 per liter. This major breakthrough removed the major obstacle of applying these fluids to mineral and material beneficiations. Other definitive statements, as well as several technologic and medical applications of magnetic fluids, have been recently summarized.
Magnetic Separation of the Second Kind
Rather than depend on the inherent magnetism of the particles to be separated, a new approach that magnetizes the medium itself will separate nonmagnetic particles of a broad density spectrum. To distinguish it from ordinary magnetic separation, this new type of separation will be labeled as “magnetic separation of the second kind.” The same force that attracts magnetic objects in separations of the first kind also attracts the entire separation medium in separations of the second kind, thereby creating a reactionary force of equal magnitude in the opposite direction (levitation force). This latter force can be made to segregate nonmagnetic particles according to their specific gravity. Magnetic fluid/magnetic field systems can, therefore, behave as the old-fashioned and time-honored heavy liquids. Most importantly, they are not limited to the 4.0 to 4.5 specific gravity of heavy liquids, but can be extended over the entire density spectrum, up to 21 or more (platinum has been levitated on magnetic fluids), thus qualifying them as the heavy media of the 21st century.
Separations depending on the above principle have been conducted in both the Soviet Union and the United States since the mid-1960’s. At the Bureau of Mines, U.S. Department of the Interior, a magnetic fluid acts as the separation medium, while the Soviet investigators use an aqueous solution (or melt) of a strongly paramagnetic salt such as manganous chloride for the same purpose.
Magnetic Levitation Force
A mathematical expression for the magnetically induced buoyant levitation force created in a pool of magnetic colloid placed in an inhomogeneous magnetic field can be derived from simple magnetostatic theories. The potential energy, U, of a magnetic dipole of volume V and moment ϒ = uV, in a magnetic field, H, is given by the negative of the dot product of the two vectors; thus
U = – ϒ·H = – ϒ H cos θ = – u HV cos θ…………………………………(1)
where θ is the angle between the two vectors ϒ and H, ϒ and H are their magnitudes, respectively, and u is the volume-averaged magnetic moment. If a small volume of magnetic fluid is placed in an inhomogeneous magnetic field such that the lowest value of the ambient field saturates the fluid magnetization, then equation 1 becomes applicable since u is a constant independent of H. Because the fluid magnetizes along the local field direction, θ=0, and
U = – u HV……………………………………………………(2)
Also, force is defined as the negative space rate of change of the potential function. Hence, the magnetic force per unit fluid volume, Fm, will be given by the negative gradient of the scalar function U/V:
Fm = – 1/V grad U = u grad H…………………………………………..(3)
The assumption made is that the magnetic moment, u, per unit volume of the fluid is independent of the spatial coordinates within the magnetic field, and that it is relatively constant independent of H. The force Fm, is in the same direction of the vector quantity “grad H” and tends to move the fluid towards the magnet.
In a two-phase system of large magnetic particles in a nonmagnetic fluid, the particles alone will move in the direction of maximum field (separation of the first kind). By contrast, a magnetic colloid behaves as a one-phase system and will be attracted as one unit to the magnet. The particles essentially impart their magnetic character to the continuous phase, both phases becoming magnetically indistinguishable in a true colloid. Because the fluid is constrained in its position by being confined in an immovable vessel, a reactionary force of equal and opposite sign will be created within the fluid and will manifest itself on immersed objects. This follows Newton’s third law of motion, “to every action there is always opposed an equal reaction.” A magnetic levitation force Fl will therefore be exerted per unit volume of an immersed body in a magnetic fluid placed in an inhomogeneous magnetic field. This force will be equal, and opposite in direction, to Fm, and, per unit volume of the immersed body, will be given by
Fl = µ grad H = – M/4π grad H………………………………………………(4)
where M is the fluid magnetization.
In this work, the fluid magnetization is represented by M, while the symbol u represents the magnetic moment per unit volume, sometimes called the intensity of magnetization. The two symbols are interrelated (1) by M = 4πµ. The emu unit of both quantities is the same; namely; erg per oe.cm³, or gauss.
The other hydrodynamic force exerted per unit volume of an immersed object of density, p’, is the Archimedes buoyancy force, which is equal to the weight, Pg, of the fluid displaced by unit volume of the solid, where p is the fluid-density. Because the true body weight is p’g per unit volume, the magnitude of the gravitational force, Fg, on the immersed object is (p’-p)g, and the gravitational force vector is
Fg = (p’-p)gk………………………………………………….(5)
where k is the unit vector in the vertical direction. The net force per unit volume, f, exerted on the immersed body is the vectorial sum of the magnetic levitation force and the gravitational force and is given by
F = Fg + Fl = (p-p’)gk – µ grad H……………………………………………(6)
Since “grad H” can be impressed upon the system in any desired direction, it is possible to overcome gravity by orienting the field gradient in a direction opposite to k. This means that a nonuniform magnetic field whose magnitude decreases in an upward direction, and hence whose gradient vector is directed downward, will tend to lift an immersed body upward. Similar relationships were rigorously derived by Epstein for an electrostatic field and by Rosensweig for a magnetic field by integrating the surface and volume forces experienced by an immersed body, and differentiating a modified Bernoulli equation to evaluate the pressure gradient.
The magnetization curves of magnetic colloids did not exhibit the hysteresis loop expected for ferrimagnetics or ferromagnetics, but produced instead a symmetrical sigmoid curve about the origin (fig. 1). This
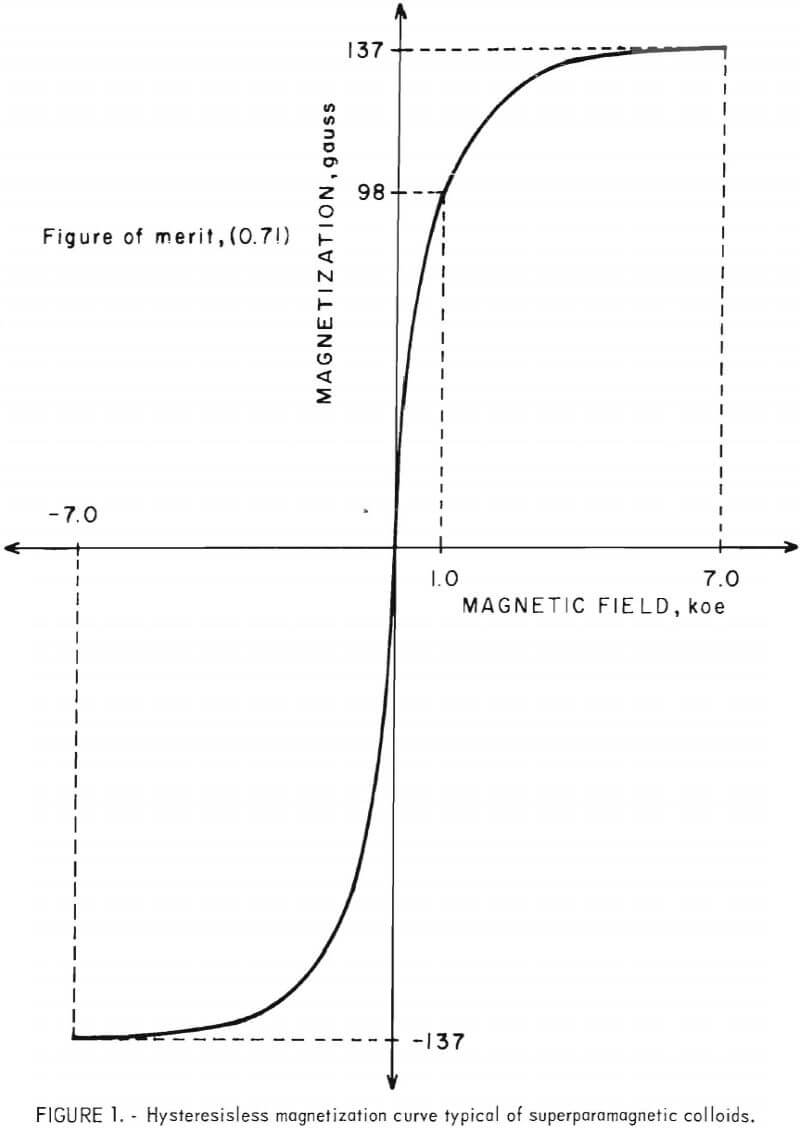
hysteresisless magnetization curve is typical of superparamagnetic substances and indicates that magnetic colloids can become magnetically saturated at reasonably attainable magnetic fields.
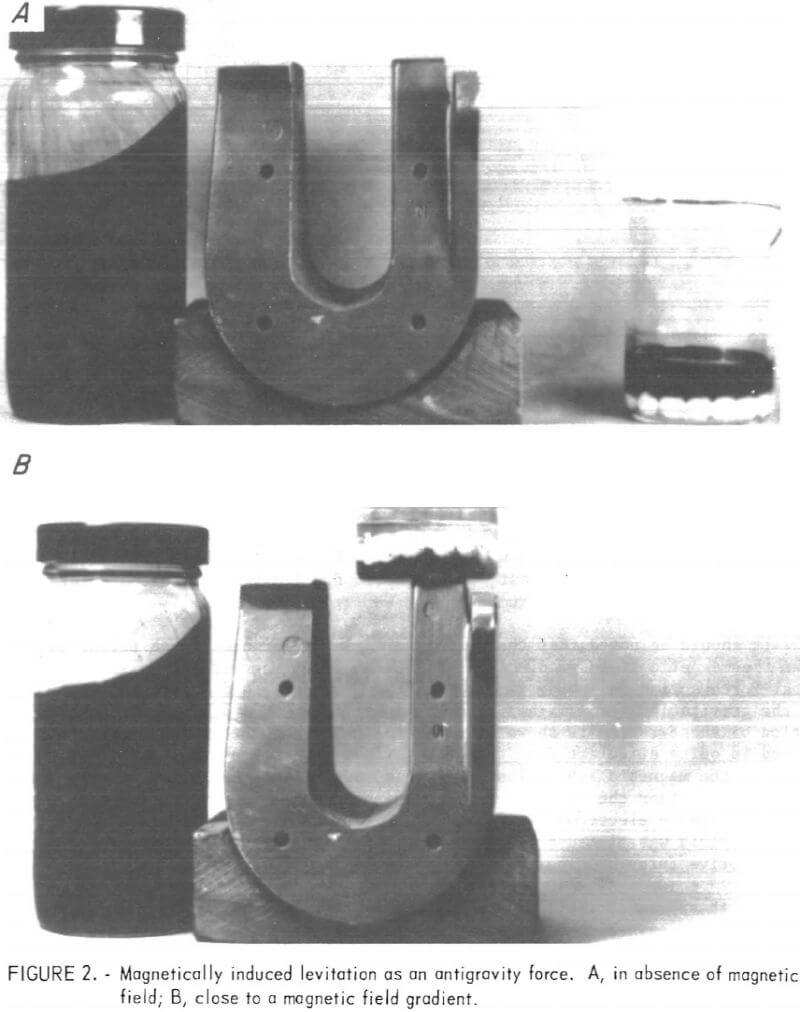
Figure 2A depicts a magnetic fluid sample of about 0.8 g/ml density containing water and some alumina beads with a density of 3.8 g/cm³. Naturally, these constituents will sink in the magnetic fluid. Figure 2B illustrates the creation of a magnetic levitation force, sometimes called an antigravity force, in a magnetic fluid-magnetic field combination. In this figure, the same container shown in figure 2A is placed on a laboratory horseshoe magnet whereby both the water and alumina are floated on top of the fluid. Thus, in a magnetic field the fluid appears to have a density greater than 3.8 g/ml. Because the fluid’s apparent density can be varied as desired by varying the magnetic field, one should be able to selectively separate particles according to their density spectrum.
Magnetogravimetric Separations
Theory predicts that the magnetic levitation force per unit volume of an immersed object is equal to the product of the fluid magnetization by the magnitude of the magnetic field gradient. One can, therefore, increase the levitating force either by increasing the fluid magnetization (stronger magnetic fluid) or by increasing the field gradient, or both.
Modern magnetic fluids can easily provide transient zero-gravity conditions when placed in inhomogeneous magnetic fields. Kaiser used an on-off magnetic field for the separation of particles (positioned at different elevations in the fluid) according to density, while Rosensweig employed the fluid as a horizontal sieve. Both methods utilized the magnetic levitation force created within the fluid in an inhomogeneous magnetic field as an anti-gravity force; that is, the former force was always oriented in the vertical direction, as illustrated in figure 2.
Figure 3A shows the principles of a continuous magnetogravimetric separation technique that orients the magnetic reactionary force in a nonvertical direction. This approach allows continuous separation of materials by means of a single magnetic fluid-magnetic field combination. The mixture is fed from a chute, and the components are received at different receptacles in the exit section. In this separation the fluid acts as a density spectrograph, diverting particles in different trajectories according to their density. A major advantage of this technique is that magnetic particles would be deflected to the back side of the separation cell and hence can be mechanically separated from the various nonmagnetic fractions.
In figure 3A, a mixture of alumina balls (white) and lead shot (black) was poured into the magnetic fluid contained in a cell that was obliquely sandwiched between two steel blocks on the poles of a laboratory magnet. The mixture instantly separated, with the components falling separately into the two beakers shown on the left side of the picture. The white alumina was partially covered with the dark fluid in the front beaker. Because of its lower density, the adhering fluid can be recovered by washing the separated
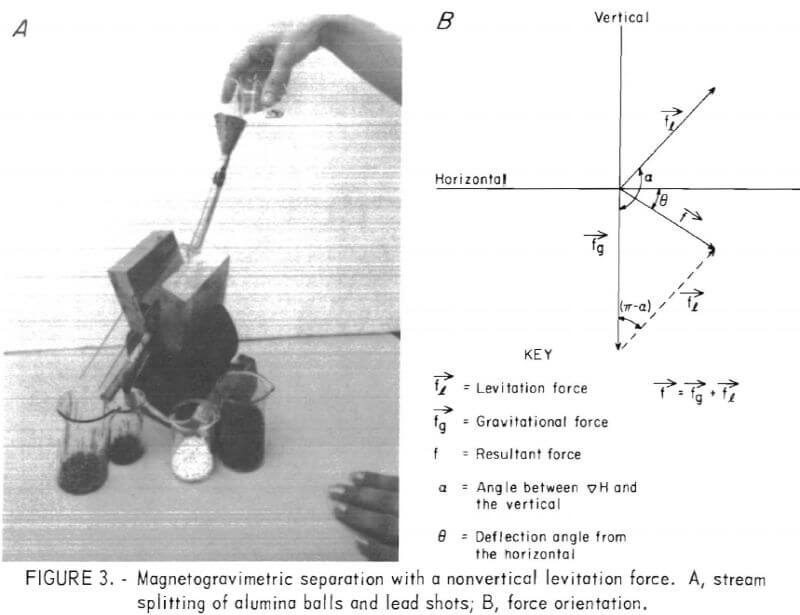
products with water. To achieve the same separation with heavy liquids, a medium with specific gravity greater than four would be needed, in which alumina would float and lead would sink. This liquid would be effective only for this separation; in contrast, the magnetic fluid can separate components over the entire density spectrum.
It should be noted that the horseshoe magnet in figure 3A was tilted to make the magnetic field gradient vector deviate from the vertical axis. The total force acting on a body immersed in the magnetic fluid is the vector sum of the gravitational and magnetic levitational forces. A diagram of the orientation of these forces is shown in figure 3B. The concept in this continuous method is quite different from that of previous separation procedures that utilized the magnetic levitation force in a vertical direction. In the latter procedures, either the magnetic field gradient was changed by use of an intermittent variable electromagnet, or a bottomless vessel containing the magnetic fluid was placed between the inclined poles of a magnet with the magnetomotive force holding the fluid in place.
The theory and principles underlying the procedure for continuous particle separation have been developed previously. This method was also successfully applied to fractionate nonferrous metals from the residue of an experimental solid waste incinerator. Screening the incinerator residue

zinc with less than 3 percent silica. Separation of this residue into its components was accomplished using a permanent magnet and a magnetic fluid with a saturation magnetization of 80 gauss (8 x 10 -3 Tesla). The light fraction collector contained 58 percent of the sample and was rich in aluminum (84.7 percent). The heavy residue was further fractionated using an electromagnet with the field adjusted to 2.4 koe and a magnetic fluid with a saturation magnetization of 215 gauss. Materials with mass density less than 7.2 g/cm³ were received in the light fraction receptacle and were rich in zinc, while the heavy fraction receiver contained material rich in copper.
The bulk of the magnetic fluid that adhered to the separated particles was recovered by floating it on water and skimming it off with the aid of moving magnets. Figure 4 is a photograph of the separator used in these bench-scale experiments and illustrates a method for returning the skimmed fluid to the separation cell.
At present, material separation processes based on magnetic fluid technology can be envisaged in reclaiming nonferrous metals from concentrated unburned urban refuse, recovering titanium and lead, and unalloyed copper and zinc from wasted nonferrous scrap, recovering lead-free zinc die-cast alloys (trade name, Zamac) from the nonferrous segments of automobile scrap, separating the gangues from native copper ores to upgrade their copper content, and recycling military wastes and scrap and processing their electronic scrap for precious metals.
Experiment
Material and mineral beneficiations were conducted on a large scale and at feed rates as high as 2 metric tons per hour (2,000 kg/hr). The following is a description of the density separation equipment, the separation procedures and the material used.
Electromagnet Powered Magnetic Fluid Separation Unit
The magnetic fluid density separation experiments were conducted using a 4.5-ton (4,000 kg) electromagnet assembly. The Walker/Magnion levitation magnet system consists of a 31.25-kw DC-regulated power supply and an electromagnet designed to provide a constant magnetic field gradient in the air gap between the pole caps. A motor-driven variable output transformer provides the capability of current adjustment from 45 to 125 amperes with a maximum output voltage of 250 volts. The power supply is rated for the regulation of the output current to better than 1 percent over the full operating range.
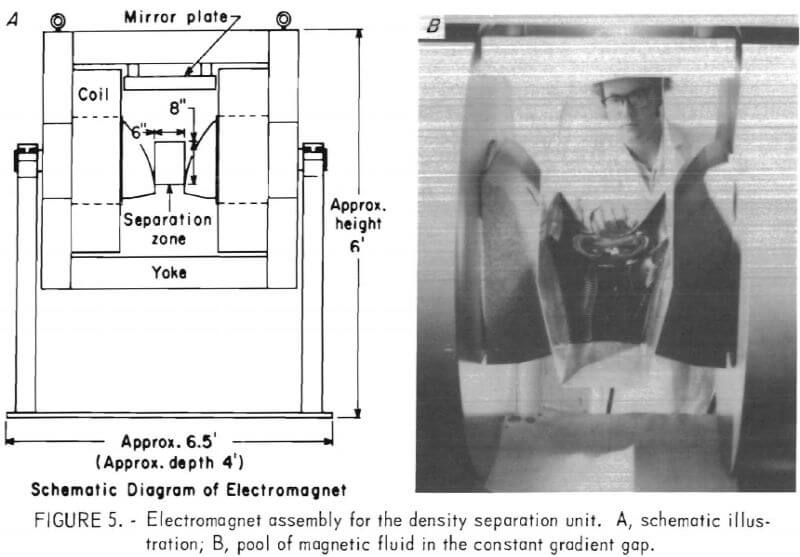
When operated at full power the lower region of the separation zone has a magnetic field of about 10 koe. To generate the magnetic field profile for density separation the special pole pieces provide a magnetic field gradient of approximately 230 oe/cm in the separation zone. A schematic drawing of the electromagnet assembly is illustrated in figure 5A, while figure 5B shows a pool of magnetic fluid held in an open-ended trough.
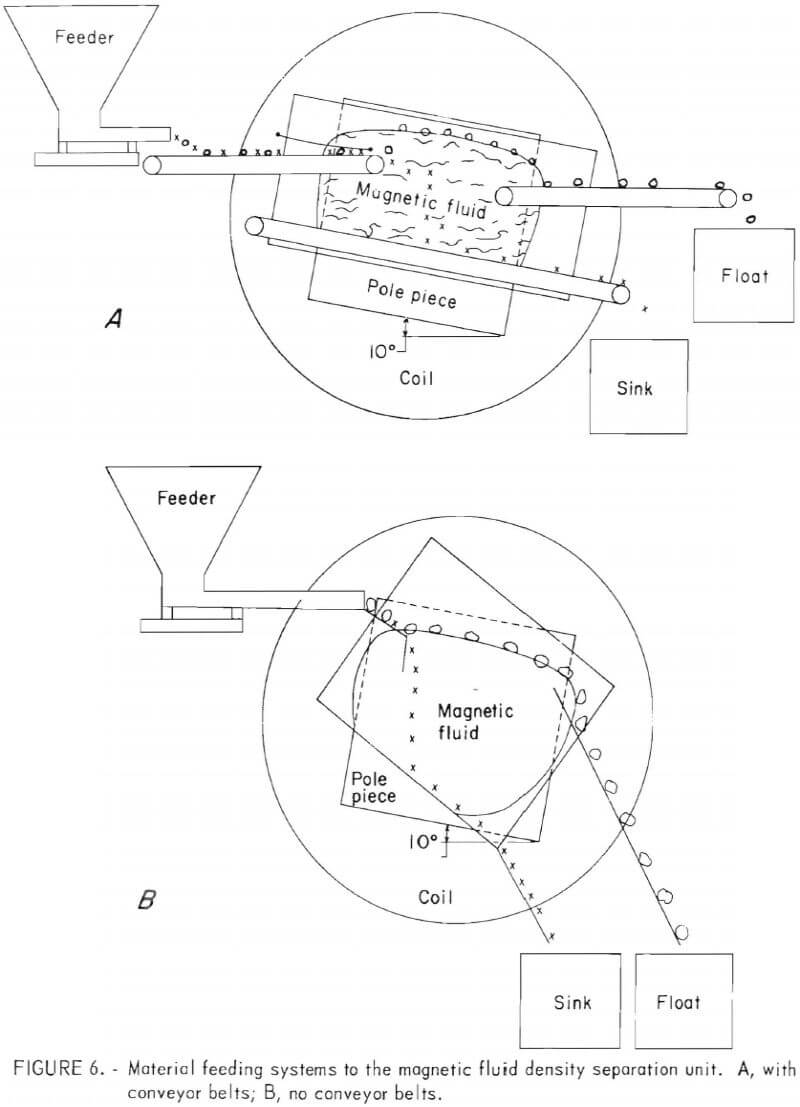
Material Feeding and Discharge Systems
Two systems for feeding the input materials into the levitation electromagnet and discharging the separated products have been devised and tested. In the first system, scrap metal is loaded into a Syntron Model SF-212 Vibro-Flow feeder having an 8-inch-wide tray. Materials from this tray are then fed onto a Dorner conveyor constructed of nonmagnetic materials and fitted with a 5-inch belt. This conveyor belt feeding system is schematically illustrated in figure 6A. A hinged deflector is used at the end of the conveyor to assist in submerging pieces of scrap into the magnetic fluid pool. As indicated in figure 6A, the magnet assembly is sloped forward so that the float product can move toward the upper conveyor for discharge. The sink product drops onto a conveyor positioned in the lower regions of the pool. Approximately 5 gallons of magnetic fluid is used to fill the open-ended aluminum trough that is mounted between the magnet pole pieces.
An alternative method of feeding materials and discharging products is shown in figure 6B. In this method the vibratory feeder previously described discharges directly onto the surface of the magnetic fluid pool. An extension was added to the feeder tray to narrow the discharge end to fit between the separation trough. The magnet assembly is sloped forward, and the float product is skimmed off using a fixed tray. When the sink products reach the sloped bottom of the separation trough, they also slide forward for collection. This second method does not give close control of the velocity of feed materials entering the magnetic pool, but it does allow the separation of materials that would tend to clog the conveyor belts.
Reagents and Materials
Separation tests were conducted in a kerosine-base magnetic fluid prepared in bulk following the Bureau of Mines peptization method. Five- to ten-gallon batches of magnetic fluid were formulated using commercial grades of iron salts, ammonium hydroxide, tall oil fatty acid, and kerosine. The reactions were completed in a 55-gallon stainless steel tank fitted with an electric immersion heater and an air-driven stirrer. Magnetic fluids with magnetization values from 100 to over 400 gauss were produced by varying the quantity of magnetite dispersed in the kerosine carrier.
The scrap metal used in this work was obtained from an automobile shredding operation. Ordinary magnetic reclamation of the major ferrous constituents left a reject containing the nonferrous and valuable metals of the automobile scrap. The rejects were further reshredded so that the bulk of the metallics was fragmented to pieces smaller than 3 inches (76 mm). The reshredding step resulted in an added benefit of pulverizing the glass and other nonmetallics, thereby facilitating their screening from the metallic portion to be segregated with magnetic fluids.
The diamondiferous concentrate was provided by a U.S. mining company, which prefers to be anonymous, having properties in South America. The minerals were mined by dredging river gravels followed by concentration by jigging. The main minerals in these beach sands were quartzite, iron silicates, goethite, and kyanite. A head sample weighing 2,750 pounds (1,250 kg) of the jig concentrate was beneficiated with magnetic fluids in presence of the mining company personnel who took the separated fractions back to their company headquarters for analysis. Twenty-two carats of diamonds (4.4 g) were recovered from the 2,750 lb of feed. One diamond was about 0.5 cm in size and weighed slightly more than 1 carat.
Two beneficiation systems were studied in this paper. The first one deals with materials recycle by segregating metals of common identity from the nonferrous segment of automobile scrap. The second system covers the upgrading of diamondiferous concentrates obtained from certain river gravels.
Nonferrous Automobile Scrap
The separation of nonferrous automobile scrap was studied with the goal of upgrading the mixed useless scrap into useful metallic groupings. The effect of the type of feeding system on the separation results was first evaluated by comparing the conveyor belt system with the direct feeding system from the vibratory feeder. The scrap metal used in these series of tests had the following approximate composition as determined by hand sorting: zinc-48 percent, aluminum-39 percent, copper and brass—7 percent, non- metals—4 percent, and less than 1 percent each of lead, stainless steel, and insulated copper wire. For the separation tests the scrap sample was pre-treated by sizing to minus 2- plus ¼-inch and removing the magnetic pieces. The composition of the float products from automobile scrap was determined as
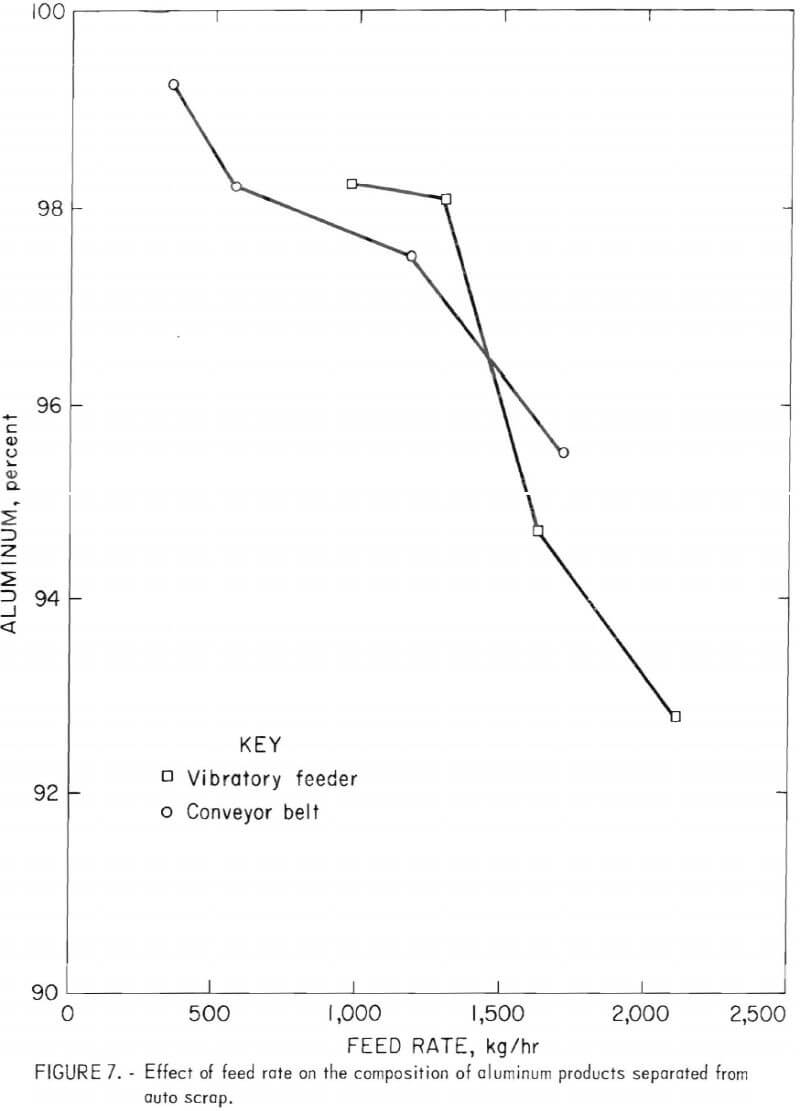
a function of feed rate using both methods of material feeding to the separator. In all these tests the separation zone was maintained at an apparent specific gravity of 4.0 using a magnetic fluid of 200 gauss saturation magnetization and an electric power setting at 70 amperes. The composition and recovery of the float product with feed rate are given in table 1. Variation of the composition of the aluminum float with feed rate is shown in figure 7 for both types of feeding systems. An inverse relationship appears to exist between the purity of the float product and the feed rate.
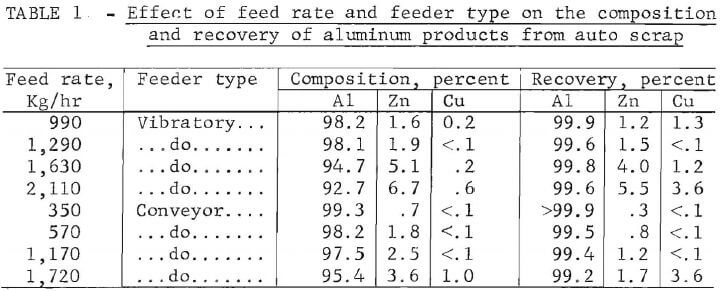
Because of the large density mismatch between aluminum and the zinc-copper fraction, it was possible to use very large feed rates and still obtain relatively efficient separations. Figure 7 indicates that the maximum feed rate for removal of aluminum depends upon the level of zinc-copper contamination the operator is willing to accept. The physical capacity of the feeder and conveyors was not reached even at feed rates as high as -2,000 kg/hr, where the aluminum fraction is still 93-percent pure while recovering 99.6 percent of the available aluminum.
The results also seem to indicate that both systems of scrap feeding were adequate for the separation of aluminum. Evidently, for aluminum at least, the velocity of the scrap pieces at the point of entry in the magnetic fluid does not influence the separation efficiency. As the density mismatch becomes smaller (for example, in separating zinc from copper), the effect of feed velocity begins to be of significance. In such cases, the conveyor belt method of feeding will be more advantageous, since feed rates with the vibratory feeder cannot be varied independently from feed velocities within the separation cell.
Tests were also conducted using a feed composed of the sink product from the aluminum beneficiation step. In these tests the separation zone was maintained at an apparent specific gravity of 7.0 (magnetic fluid saturation magnetization of 400 gauss and electrical power supply setting at 70 amperes). With this configuration, the zinc fraction was separated from the heavy metals: copper, stainless steel, and lead. Because the density mismatch is now greatly reduced, the composition of the zinc product exhibits greater dependency on feed rate than observed when separating aluminum. The results of these tests are given in table 2.
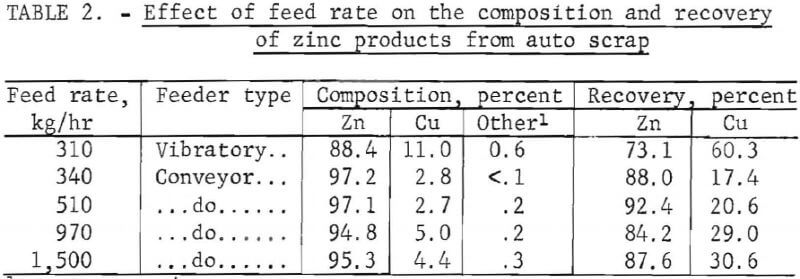
The composition of the products listed in tables 1 and 2 was determined by hand sorting of the metals into their specific groupings. The purity of the zinc product was considerably better when the conveyor belt was used to feed the auto scrap instead of the vibratory feeder. The vibratory feeder gave inefficient separations of zinc, even at the smallest feed rate as shown in the first row of table 2. Comparing the first and the last row of this table indicates the desirability of using the belt feeder. A higher purity zinc product with greater recovery was obtained with the conveyor belt even though the feed rate was five times faster than with the vibratory feeder.
Upgrading Diamondiferous Concentrates
The material used in this phase of investigation consisted of a jig concentrate produced from river gravels dredged in South America. At present the jig concentrates are carefully ball milled for about 60 hours to crush the softer gangue minerals. Meticulous crushing of the jig concentrate is important to preserve the precious stones intact and to avoid their catastrophic degradation through size reduction. The mill contents are then screened and hand sorted to recover the diamonds. To eliminate the energy- and time¬consuming grinding method, the magnetic fluid beneficiation technology was attempted as an alternate route.
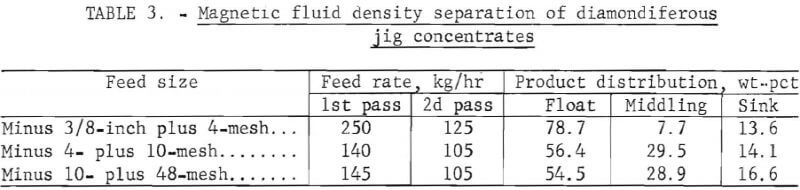
The diamondiferous jig concentrate sample was screened into the three size fractions of minus 3/8-inch plus 4-mesh, minus 4- plus 10-mesh, and minus 10- plus 48-mesh with 9, 48, and 43 weight-percent, respectively, reporting to each size fraction. Total combined weight of the sample was 1,250 kg. Preliminary testing on the jig concentrates indicated a problem with the conveyor belts clogging with fines. Therefore, density separations were conducted with the feeder and product discharge equipment without conveyor belts, as illustrated in figure 6B.
Several magnetic fluid and power supply settings were initially tried in attempts to develop a two-stage separation scheme. In this scheme the first pass would get rid of the light minerals and sands by floating them away.
This is followed by a second pass to produce a middling product containing the diamonds floated from the heavier minerals. To aid in determining the proper separation settings, colored cubic-shaped tracers of specific gravity 3.5 from J. E. Encapsulations, Essex, England, in the same size ranges as the feed, were used to monitor the results. The final choice consisted of a magnetic
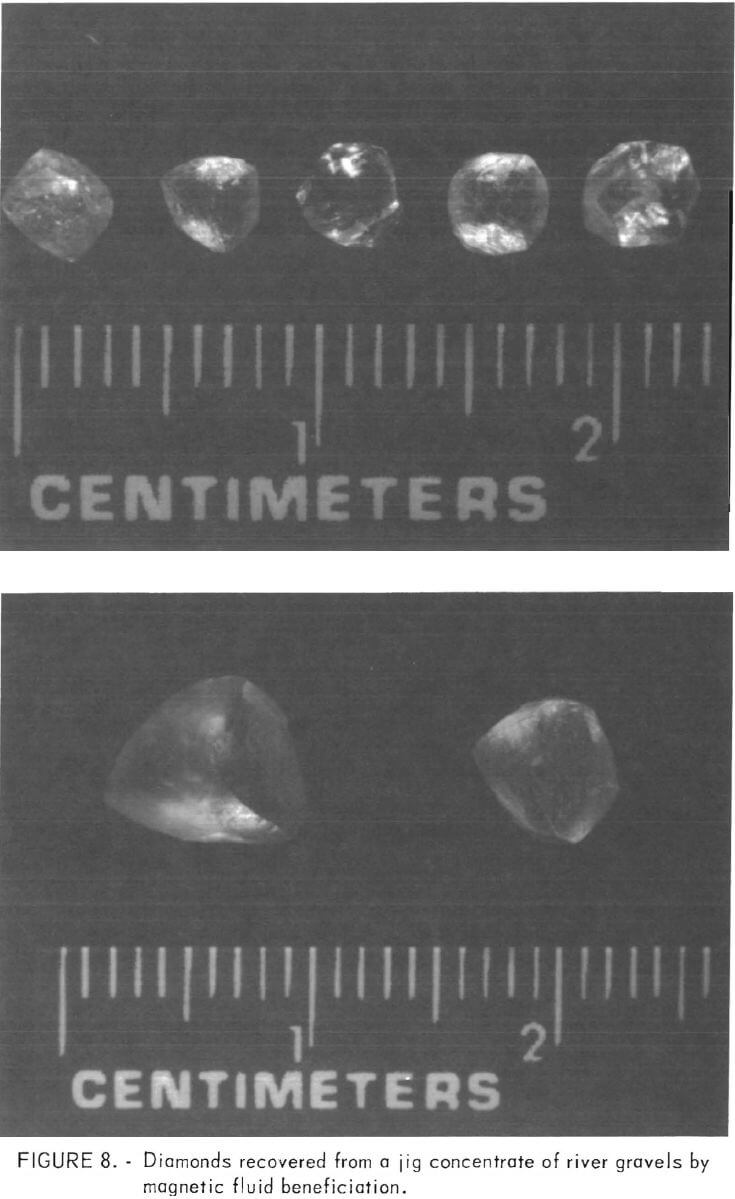
fluid of 130 gauss along with a power supply setting at 65 amperes to float the light gangue minerals from the diamonds. For the second pass through the separator, the magnetic fluid was not changed, but the power supply was adjusted in a range of 100 to 110 amperes to float the diamonds from the heavier gangue minerals. The higher power setting was used when separating the coarse feed. Use of the same magnetic fluid for both passes eliminated the need to wash the first sink product before the second processing step. The results of these separation tests are listed in table 3. Figure 8 shows some of the diamonds that were visible in the float product from the second pass.
Following the separation tests the products were returned to the mining company. After hand sorting they reported that all of the diamonds in the minus 3/8-inch plus 4-mesh fraction had reported to the middling product. The finer sizes were sorted as a group, and more than 90 percent of the diamonds were found in the middling product.
The data in table 3 indicate that diamonds were concentrated with magnetic fluids into a product containing about 8 and 30 percent, respectively, of the starting weight for the coarser and finer sizes. By rejecting over 92 percent and 70 percent of the gangue in these two cases, it became possible to hand sort the precious stones as well as eliminate the energy-wasting, time-consuming, and catastrophically destructive grinding steps.
Summary and Conclusions
The principles underlying the magnetogravimetric separation technique with magnetic fluids have been outlined. The technique has been applied to two main beneficiation areas involving material recycle and mineral recovery. Two operational modes of material feeding were compared for the separation of metals from nonferrous automobile scrap. Recovery of aluminum was excellent using either a conveyor belt or a direct feeding mode. Separation of zinc from heavier metals was improved where a conveyor belt system was used.
Approximately 70 percent of the gangue minerals were removed from diamondiferous jig concentrates, while all the diamonds were recovered in the coarser fractions. Although these separations were conducted on a relatively large scale, commercial application would be more attractive by achieving larger throughputs. Beneficiations based on these techniques would therefore benefit from the rapidly evolving technology of superconducting magnets.
Methods have bean devised to clean up rhe separated products from the adhering separation medium, and to recover the latter for recycle to the separation cell. These methods will be the subject of subsequent publications. Although significant progress has been achieved at the Bureau of Mines in the production of kerosene-based magnetic fluids, new and economic methods of producing other magnetizable media are highly desirable. Among these media, water-based magnetic fluids may improve the process economics by eliminating the need to dry the feeds and by simplifying the media recovery procedure.
The Bureau of Mines has used colloidal solutions of magnetite in a magnetic field to segregate non-magnetic materials such as non-ferrous scrap metals and to concentrate precious minerals. Conventional magnetic separation relies on the inherent magnetic susceptibility of the material to be separated and may be designated as magnetic separation of the first kind. When the medium of separation rather than the separated particles are made magnetizable, a new system of gravity separation results. In this new magnetic separation of the second kind, a magnetic fluid rather than air or water acts as the separation medium. The same force that attracts magnetic objects in separations of the first kind also attracts the entire separation medium in separations of the second kind, thereby creating a reactionary force of equal magnitude in the opposite direction. This force, sometimes called the levitation force, can be made to segregate nonmagnetic particles in a flowing stream according to their specific gravity. The technique has been applied to segregate nonferrous metals from automobile and appliance shredders and mixed scraps. The system has also been used to upgrade diamondiferous jig concentrates from certain river gravels in order to facilitate the sorting and recovery of their diamond content.
