Table of Contents
Formation and Transport of Acid or Toxic Leachate
The factors which control the quantity and quality of leachate formed from mining wastes are many and complex. These factors include not only the physical and chemical characteristics of the waste, but also climatological, hydrological, geological, and biological factors. The method of waste disposal and the selection of the disposal site are also determinative factors to the extent that they influence the availability of oxygen and water within the wastes. As will be discussed, the availability of these substances is essential for formation of an acid drainage or leachate.
The discussion presented in this section has two purposes. First, it introduces the factors considered in designing the waste leaching experiments conducted during this study. (These experiments, which were intended to closely simulate real leaching conditions over a long period of time, are described in Section 5 of this report.) Second, this discussion provides a background for interpretation of the leaching experimental results and for evaluation of the long-term potential for contamination of ground water by mining-waste leachates.
The production of acid and toxic leachates from mining wastes is considered to be the result of the oxidation of iron sulfide minerals contained in these wastes. The most common of these minerals is pyrite (FeS2). For this reason, the pyrite oxidation process has been intensively investigated in an effort to identify the factors which control it. The discussion which follows summarizes the major findings of these studies.
While considerable attention has been given to the processes which result in the production of acid from mining wastes (that is, oxidation), little attention has been given to the leaching of metals contained in these wastes, or to the mode of transport of waste components from the waste disposal area. The current state of knowledge regarding these last two factors is also reviewed in this section.
Pyrite Oxidation Model
The acid-forming materials present in many mine wastes are the iron sulfides, the most common of which are pyrite (FeS2), marcasite (FeS2) , and pyrrhotite (Fe5S6 to Fe16S17). The acid-producing potential of these minerals is greatly increased in an oxidizing environment, such as is generally found in mine waste dumps, abandoned tailing disposal areas, and coal refuse piles. When exposed in this manner, these minerals decompose by a complex series of chemical reactions. The products of this decomposition are easily dissolved in water to produce acid and associated hydrous iron complexes.
Of the mineral forms identified above, pyrite is by far the most abundant. For this reason, the pyrite oxidation process has been intensively investigated. While it is likely that the factors involved in this process are common to all the iron sulfide minerals, the review which follows focuses primarily on pyrite oxidation.
Pyrite occurs in ore bodies and coal strata as enhedral crystals, coarse-grained (greater than 25-micrometer) masses which replaced original plant matter, coarse-grained platy masses occupying joints in the strata, and framboidal clusters of spheres of pyrite measuring about 0.25 micrometer in diameter and finely disseminated throughout the ore or coal and associated host rock or strata. Of these four basic types, it is only the last one, the framboidal type, that decomposes rapdily enough to produce a severe acid drainage or leachate.
The mechanism which makes framboidal pyrite more reactive than other forms is not well understood. Since massive pyrite does not become more reactive when crushed, size alone is not the determining character. The possibility that the various types of pyrite may contain trace metals which could differentially catalyze their oxidation has also been investigated and eliminated. A survey of the mineralogical literature has revealed that the various crystal faces (forms) of pyrite have different oxidation resistances, the octahedron being the most susceptible to attack. Researchers studying the mineralogical character of pyrite have also attributed differences in observed rates of oxidation of two forms of pyrite to measured differences in surface area and pore-size distribution.
The observation to be made on the basis of the above is that not all forms of pyrite will react at a sufficiently fast rate to produce a severe acid condition. Furthermore, although a mechanism to explain the difference in reactivity has not been delineated, it does appear that textural differences (that difference in surface area and crystal structure) are the major factors influencing reactivity of the various pyrite types.
The chemical reactions and factors governing the rates of these reactions have been intensively investigated during recent years. It has generally been acknowledged that the chemical oxidation of pyrite in the presence of water and air, to a soluble hydrated iron sulfate, and the subsequent dissolution and hydrolysis of the oxidized form in water, is responsible for the generation of acid and the concentration of iron and sulfate found in drainages and leachates from mining wastes. This oxidative process is described by the following stoichiometric reactions:
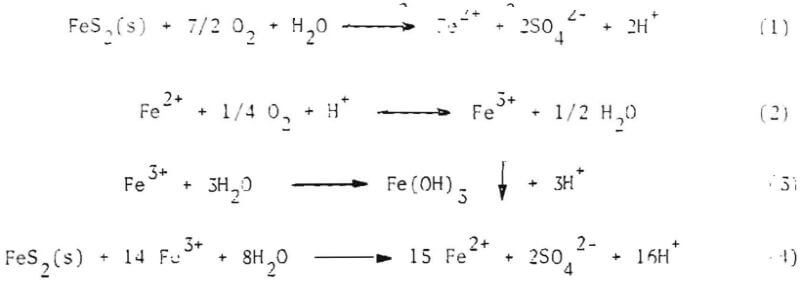
In the above reactions, FeS2(s) is symbolic of the various iron sulfite minerals which may occur in different stoichiometries but always contain the same ratio of sulfur to iron.
A review of the above reactions reveals that, for every mole of iron disulfide (hereinafter referred to as pyrite) consumed, four equivalents of acidity are produced-two equivalents from the oxidation of S2-, and two from the oxidation of Fe2+. These reactions also illustrate that pyrite may be oxidized by both oxygen and ferric ion (Fe3+) .
While some controversy and disagreement exist in the literature as to the relative significance of pyrite oxidation by O2 and Fe3+, in real pyritic systems, it is useful here to review the major findings of these studies to gain a clear understanding of the physical, chemical, and biological factors which have been found to control the rates of oxidation by O2 and Fe3+ in the laboratory.
The approach that has been taken by one group of researchers has been to conceptualize the factors controlling the oxidative and leachate formation processes in terms of rate processes. These processes include: (a) rate of transport of reactants to the points of reaction; (b) rate of the reactions themselves, or reaction kinetics; and (c) rate of transport of the products away from the points of reaction. The factors which govern the transport of pollutants are discussed later in this section. The major findings, to date, for the first two rate processes identified above are:
- The amount of oxygen dissolved in water entering a natural system supplies only an insignificant portion of the total demand for oxygen required at observed rates of pyrite oxidation. Therefore, to support these rates, oxygen must enter the system as a gas. Furthermore, oxygen transport within the system must also be predominantly in the gas phase, because the rate of diffusion of oxygen in water is extremely slow, and pyrite covered by more than a centimeter or two of water will be effectively shielded from exposure to oxygen. Therefore, the factors which control the transport of O2 (that is, forced draft [convection] or molecular diffusion) will determine its availability for reaction at the pyrite surface.
- The O2, and Fe3+ pyrite oxidation modes are independent of one another. For a given pyrite surface, oxygenation rate is, for all practical purposes, dependent only on the oxygen concentration in the aqueous phase surrounding the reactive site on the pyrite surface and is independent of other solute concentration. Ferric ion (Fe3+) oxidation is primarily determined by the ferric/ferrous ratio (Fe3+:Fe2+] and the free ferric ion concentration, and is not affected by dissolved oxygen.
- Kinetics for the oxygenation of pyrite have been experimentally determined as a function of (a) temperature; (b) oxygen concentration; (c) pH; (d) water partial pressure; (e) surface area (or texture) of, the pyrite; and (f) concentration of iron, sulfate, and other ions. Specifically, the rate of oxidation has been determined to increase with increasing temperature, oxygen concentration, pH, percentage of relative saturation. At the pyrite surface, and surface area. Concentrations of iron, sulfate, and other ions have been found to have a negligible effect at normal pils.
- Kinetics of pyrite oxidation by ferric ions have been determined as a function of (a) ferric/ ferrous ratio, (b) total iron concentration, and (c) free ferric ion concentration and pH. Specifically, the rate of pyrite oxidation increases as the ferric/ferrous ion ratio, total iron concentration, and free ferric ion concentration increase. The significance of the ferric/ferrous ion ratio is a result of the much greater adsorption equilibrium constant exhibited by pyrite for ferrous ion. Therefore, the reaction sites on the pyrite crystal are preferentially occupied by ferrous ions, and ferric ions gain a competitive advantage for reaction sites only as the ferric/ferrous ion ratio becomes greater than 1.0 As the pH increases, the ferric ion oxidation rate becomes limited by the (free) ferric ion concentration in solution and would be negligible at pHs greater than 4.0
- Acidophilic iron oxidizing autotrophs can, under favorable conditions, increase (that is, catalyze) the rate of pyrite oxidation by maintaining a high ferric/ferrous ion ratio in the solution surrounding the pyrite surface. The rate of production of ferric ions by the cells is dependent on the total number of active cells in close enough proximity to the pyrite to allow diffusion of the ferric ion back to the pyrite surface. The organisms reach a limiting concentration of 10 8 – 10 9 cells/milliter, and the degree of bacterial catalysis is thus dependent on the volume of water per unit of exposed pyrite.
- In a real system, bacterial activity is undoubtedly limited by pH and oxygen concentration, as well as by nutrient levels. In addition, some researchers believe that the buildup of the products of pyrite oxidation (that is, salts and acid) may also severely limit bacterial activity in the thin film of water surrounding the pyrite surface. This factor has not yet been investigated and is the cause of most of the, disagreement and controversy regarding the relative significance of the Fe3+ oxidation mode.
- At present, there is sound evidence for the occurrence of significant bacterial catalysis of pyrite oxidation only in environments such as arc found at the top surface of refuse piles, dry (that is unsaturated) tailings, and spoil banks.
Mobilization of Metals
Most of the heavy metals associated with mining wastes and of environmental concern are thought to occur primarily as sulfide minerals. These metal sulfides are subject to the same oxidative processes as is pyrite.
For exampie, air oxidation, biological oxidation, and Fe3+ oxidation of metal sulfides have been cited. A notable difference between oxidation of the metal sulfides and oxidation of pyrite is that acid is not produced during metal sulfide oxidation.
In a pyritic mining waste system, the generation of acid that accompanies pyrite oxidation would be expected to greatly increase the solubilities of the metal present. Therefore, in addition to mobilization by the normal oxidative processes when mining wastes are disposed of on the surface, the generation of acid by oxidation of pyritic materials creates an acid environment which greatly accelerates the dissolution of metal sulfide or oxide minerals present.
Acid and Metal Transport Model
The transport process of ultimate concern is the rate of transport of pyrite oxidation products (that is, iron, H+, and SO4 2-) and solubilized metals away from the reaction site to a receiving stream or ground-water system. Initially, the oxidation products may either diffuse along water films to a storage area or, because of the hydroscopic nature of the oxidation products (that is, salts), condense water from the surrounding air to promote seepage. It is not likely that either of these transport modes will result in significant movement of pollutants from a waste disposal area.
However, when intermittent percolating rain water or a rising ground-water table intersects the area of oxidation-product and solubllized-metal storage, these pollutants will be flushed from the waste disposal area.
The transport model described above is supported by study and observation of real mining waste systems. Data collected during an investigative study of a coal refuse pile located in Illinois indicate that the pyrite oxidative process occurred only at the surface of the pile exoosed to the atmosphere, the zone of oxidation extending only a few inches into the pile. Results of this study further indicate that the pyrite oxidation reaction was more or less constant with time, with the acid products accumulating in the outer, reactive mantle of the pile. When a storm occurred, approximately 70 percent of the acid salts appeared in the runoff, and the remainder was carried into the interior of the pile, eventually reappearing as seepage.
The “flushing” phenomenon described above has also been observed in abandoned metal-ore tailing disposal areas in Colorado. The spring snow melt in the mountainous areas has been observed to flush heavy loads of solubilized metals from these tailing areas.
Similarly, other studies have demonstrated that the amount of contaminants which can be generated and flushed from refuse piles or stored coal piles is dependent upon the reaction time available within the waste pile before the contaminants are flushed from the pile by rainfall. The following observations are also pertinent:
- The time required to complete the flushing of contaminants from the coal pile is dependent upon the volume of water applied to the pile and the duration of the application.
- Before flushing is complete, concentrations of contaminants are inversely proportional to the coal pile leachate.
- After flushing is complete, there is relatively no change in contaminant concentrations with changes in flow rate. With continuing heavy flows, some additional dilution of the minimal concentrations experienced after flushing is complete is anticipated.
To summarize, it is reemphasized that, if the mining waste is fully saturated with (or submerged in) water, very little oxidation of pyrite or metals will occur, and, subsequently, little acid production or solubilization of metals will occur. However, when mining wastes are exposed to the atmosphere, oxidation will occur. As this process proceeds, the oxidation products (that is, acid and solubilized metals) accumulate in the waste. Subsequently, when rainfall or snow melt occurs, these products are flushed out of the waste disposal area and either enter surface drainage as runoff or percolate through the strata below the waste pile into a ground-water svstem.
Field Sampling and Laboratory Waste Leaching Program
Scope of Section
The methods used during this study for selection of sample sites, collection of field samples, and waste leaching in the laboratory are described in this section.
Site Selection
The primary goal of the site-selection process was to select mining operations representative of most of the coal mining industry. To meet this objective, sites were selected in each of the three major coal mining regions of the United States. Within each of these regions, sites were preferentially selected from major coal producing states. Finally, within each state, an effort was made to select sites located on major coalbeds. The 20 leading coalbeds in terms of production during 1977 are described in table 5-1.
Additional objectives for site selection were choices of both surface and underground mining operations, operations which employed tipples only, and operations which employed washing plants for more extensive coal cleaning. Finally, an attempt was made to select operations in both areas which mine “clean” coals and areas which mine coals that are noted to be higher in sulfur and somewhat more mineralized. In general, coal mined in the Western coal mining region is noted to be cleaner than coal mined in either the Eastern Interior or Appalachian coal mining region. However, within these latter two regions, the situation with respect to sulfur content and mineralization of coal is highly variable and localized. This situation can also be expected to apply to coal mining wastes.
On the basis of the criteria outlined above, 11 sample-collection sites were selected. Descriptions and locations of these sites are presented in table 5-2. Permission for access to each of these sites was formally obtained from the appropriate mining companies in advance of the field sampling effort.
Sample Collection
Thirty-two coal waste samples were collected from a broad range of coal mining regions. These samples included waste rock, overburden, and preparation-plant refuse. In some instances, samples were also collected from coal stockpiles to evaluate this potential source of leachate.
The procedures which were followed during the field collection of the coal mining wastes were adopted from the following ASTM designations:
- ASTM D75, Standard Methods of Sampling Aggregates.
- ASTM D2234, Standard Methods for Sampling Coal.
- ASTM D2687, Standard Methods of Sampling Particulate Ion-Exchange Materials.
In every case, an effort was made to acquire samples of fresh waste material as recommended by ASTM D75. Samples of coal preparation-plant or tipple refuse were collected either from refuse bins at the preparation plant or from truck loads of refuse recently dumped at the disposal site. In the latter case, samples were always collected from the discrete piles left by dump trucks, and not from areas in which the refuse had already been spread and compacted by bulldozers, etc. To acquire as representative a sample as possible, each refuse sample was composited from increments collected from 8 to 12 different truckloads of refuse.
In many cases, fine refuse discarded in slurry form to a settling pond was sampled from delta accumulations of the refuse within the pond or from banks of refuse which had recently been bulldozed or dredged from the pond for subsequent disposal by other means. In either case, the sample was composited by collecting increments from 8 to 12 different locations within the pond or refuse bank.
Freshly disturbed overburden was also collected by compositing methods. In some cases, the various strata in the overburden had been mixed, and samples of mixed overburden were collected by compositing increments from 8 to 12 different locations on the overburden pile. In other cases, coaly shale or suspected acid-producing material had been segregated from other overburden strata for subsequent burial during reclamation. Where this practice was encountered, separate samples of both the coaly shale and other (mixed) overburden strata were collected.
In a few instances, aged refuse and stockpiled coal samples were also collected using the compositing procedure described above.
Subsequent to the collection of each sample, the composite was placed on a plastic sheet and mixed and divided using the standard quartering procedure described in ASTM D2687. Alternate quarters were subsequently used to comprise the sample required for laboratory leach testing.
Immediately following collection in the field, each sample was placed in a moisture-tight plastic bag and labelled. These bags, in turn, were packed in ice in ice chests and shipped to the laboratory.
Leach Testing Methods
Approach
The coal waste samples collected during this study were leached by three different methods. These methods include a column technique that was developed during this study to simulate real-time waste leaching by simulated rainwater; the Environmental Protection Agency (EPA) extraction procedure developed by that agency to identify hazardous wastes; and an American Society for Testing and Materials (ASTM) procedure that was developed by a panel of scientists and industry representatives as a proposed alternative to the EPA extraction procedure. It was desired to compare the results of these three methods to determine their relative accuracy and practicality in predicting the leaching characteristics of mining wastes under normal conditions. These three leaching methods are dissimilar in many respects, as indicated by the descriptions which follow.
Column Leaching Method
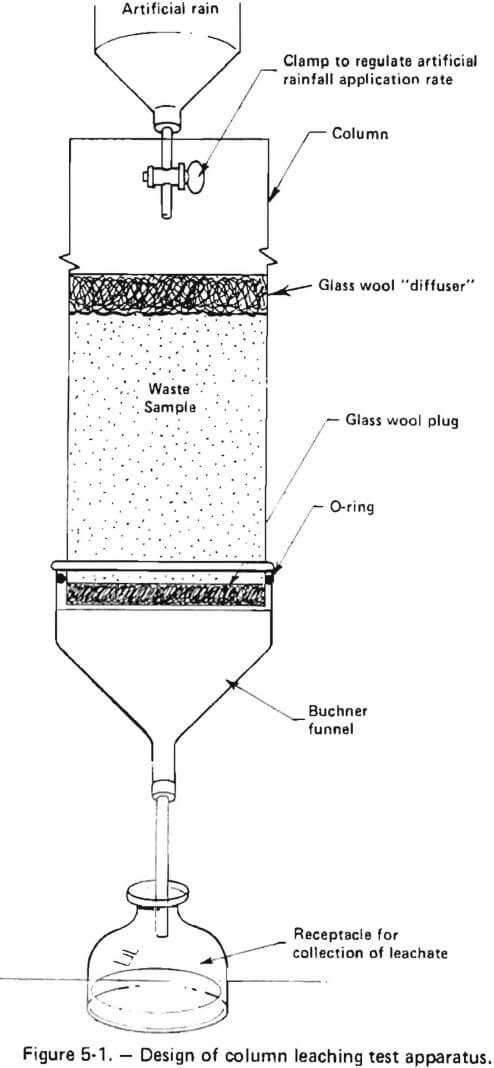
The column method developed for use during this study was designed to simulate real-time leaching of coal wastes using artificial rainfall formulated to reproduce regional precipitation chemistry. The coal waste was loaded into a vertically supported PVC (polyvinyl chloride) column to allow simulated rainfall, added at the top of the column, to slowly percolate through the waste material. This design allowed leaching of the waste under non-saturated conditions and simulates real field conditions in overburden spoils or refuse pile
The volume of simulated rain added was designed to represent the annual normalized precipitation for the geographic region in which the waste sample was collected. This resulted in “rainwater” to sample ratios ranging from 1:2.8 (milliliters:gram) to 2.6:1 (milliliters:gram). In addition, since most snow melt and rainfall occurs during a 4- to 7-month period in coal mining regions, it was considered realistic to add a 1-year normalized precipitation volume to the columns during the 5-month period of the column leaching experiment. During this 5-month period, the artificial rain was applied at intermittent intervals to again simulate real field conditions of alternate wetting and drying periods.
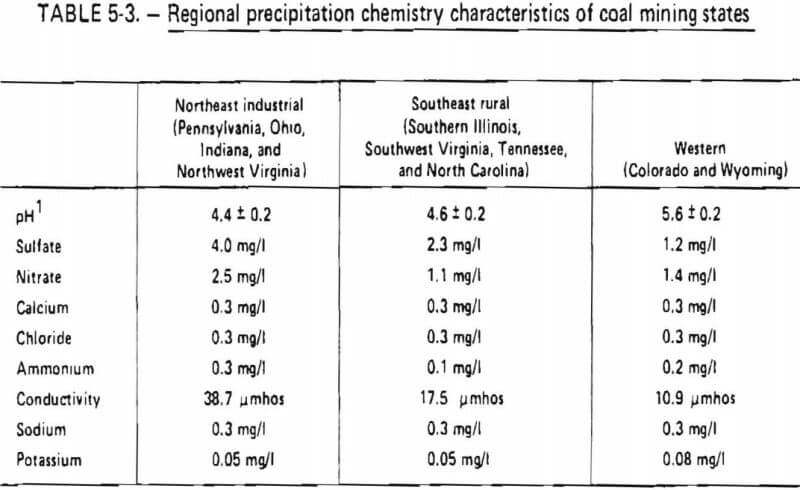
The leaching solutions used in the column leach test were formulated to chemically simulate various types of rainwater. The formulae employed were derived on the basis of data available from three major precipitation chemistry monitoring networks. Three formulae were derived on the basis of distinct regional differences in rainfall chemistry. Average regional precipitation chemistry characteristics are shown in table 5-3.
Leachate which drained from the columns was collected and filtered through a 0.45 micrometer Millipore filter prior to analysis. Sample splits collected for later metal analysis were preserved with nitric acid to below pH 2. All other analyses were performed immediately after leachate collection.
The column design which was employed is depicted in figures 5-1 and 5-2. A detailed description of the column test method is presented in Appendix A. The rationale for use of an annual normalized precipitation volume is presented in Appendix B.
EPA Extraction Procedure
The EPA extraction method is intended to generate an aqueous solution for evaluation of readily extractable materials in waste under acidic conditions. Wastes used in this test are not necessarily tested in the physical form in which they are disposed. The method specifies grinding the material, if necessary, to facilitate passing it through a 3/8-inch sieve. However, for comparative purposes and to eliminate any bias due to sampling, only the minus-3/8-inch fraction of each waste sample was used, and grinding was not practiced during this study.
The extraction procedure consisted of combining the sample in a 10:1 weight-to-weight ratio of deionized water to sample. The pH of the solution was then adjusted to pH 5 with 1:1 acetic acid. The sample slurry was mechanically stirred for a period of 24 hours, with the pH maintained at 5.0 ± 0.2. After 24 hours, deionized water was added to obtain a 20:1 dilution ratio, and the slurry was subsequently filtered through a 0.45-micrometer filter. The filtrate was preserved with nitric acid to less than pH 2, and analysis of metals was subsequently performed. A detailed description of this method is provided in Appendix C.
ASTM Leach Test (Water Shake Extraction Procedure)
The ASTM leach test used is intended as a means of generating an aqueous solution for evaluation of readily extractable materials in wastes. It is specifically designed to determine collectively the time-dependent diffusion-controlled and surface-washing contributions to leachate from the waste tested. Wastes are typically tested in the physical form in which they are discarded, thereby reducing bias resulting from such sample-preparation methods as crushing and grinding.
In simplest terms, the method consists of agitating the sample in a 4:1 (milliliter:gram) ratio of Type IV reagent water to sample. Samples are agitated in a mechanical shaker for 48 hours, followed by separation of the solid and liquid phases by filtration through a 0.45-micrometer membrane filter. The filtrate is preserved by methods appropriate to the analyses to be performed.
A detailed description of the ASTM procedure is provided in Appendix D.
Methods for Chemical Analysis of Leachates
The chemical parameters selected for analysis during this study are listed in table 3-4. The metals identified as priority metals have previously been identified as the metals which are generally of greatest environmental concern
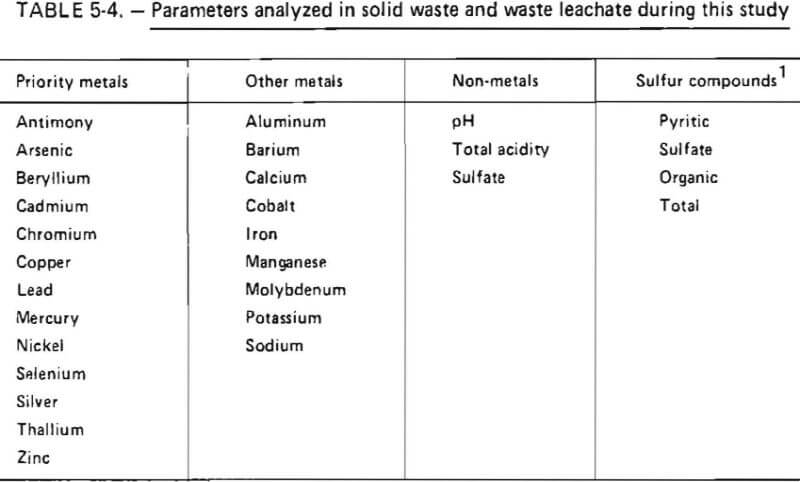
at present. The other metal parameters listed were selected on the basis of their known association with coal and coal-waste materials. The sulfur content of each waste sample was determined because pyritic sulfur is accepted as being the principal acid-producing material present in mining wastes. The extent to which this material oxidized to produce acid was monitored by measurement of the total acidity and sulfate concentrations in the leachate as well as the leachate pH.
The metal content of both the solid wastes and the resultant leachates was analytically determined to provide a basis for determination of the extent to which the various waste components were leached.
The procedure employed for analysis of the metal content of each solid waste consisted of first grinding the minus 3/8-inch fraction of the waste (with a ceramic mortar and pestle) to a fine powder. One gram of the powder was mixed with 10 grams of concentrated nitric acid, and the solution was boiled to dryness. The residue was digested a second time with nitric acid, and approximately 5 grams of concentrated hydrochloric acid was then added to the residue and boiled to dryness. The final residue was mixed with 100 milliliters of deionized water and the supernatant analyzed for metal content by atomic-absorption spectrophotometry. (Refer to table 5-5.)
The digestion procedure described above did not result in the complete digestion and dissolution of all the material contained in the mining wastes, the inert material present probably consisting of siliceous minerals, etc. Therefore, it is unlikely that this digestion resulted in a total extraction of all the metals present, since some metal was undoubtedly bound within the matrix of the inert material. For this reason, the analysis of the solid wastes is most accurately described as a “total extractable” analysis, rather than as a “total” analysis in the sense which implies complete destructive analysis. Therefore, the metal content of waste samples reported in this study should be considered to be the maximum leachable concentrations of metals contained in these wastes.
Metals in leachate samples obtained during leach testing were also analyzed by atomic-absorption spectrophotometry.
Non-metal parameters of leachates were analyzed by either titrimetric or turbidimetric methods, as identified in table 5-5.
Results of Laboratory Waste Leaching Experiments
Purpose and Scope of Section
One purpose of this study was the development of a laboratory leach test which would simulate long-term leaching of coal wastes by rainwater under field conditions. The method ultimately developed (that is, the column method) has been described in the previous section. In this section, the degree to which this method simulates real field leaching conditions is evaluated on the basis of the leach test results obtained during this study, compared to results of previous studies of coal waste leaching under actual field conditions as reported in the literature. The results obtained by this method are also compared to the results obtained by leaching the same coal wastes by the EPA and ASTM leaching procedures previously described (in Section 5) to determine the extent to which these latter two methods accurately predict the leachability of coal wastes.
Evaluation of Column Leach Test
As discussed in Section 4, the processes governing the leaching of mining wastes are primarily those which govern: (1) oxidation of pyrite, and subsequent acid production; (2) weathering of mineral components and leaching of trace metals; and (3) dissolution of salts contained in the mining wastes. When pyrite is present, its oxidation and subsequent production of acid will be the predominant factor which influences the leaching of trace metals. Therefore, the column leach test developed during this study was designed to simulate the factors which influence the oxidation of pyrite.
One measure of the extent to which the column leach test design was successful in simulating real field conditions is the extent to which the leachates obtained during this study compare with actual seepage and drainage from coal wastes. Summaries of the compositions of coal waste leachates experimentally produced by column leaching during this study are presented in tables 6-1, 6-2, and 6-3. The chemical characteristics of actual coal and coal-waste leachates (that is, seepage and drainage) as previously reported in the literature are presented in table 6-4. The waste samples collected during this study were not collected from exactly the same sites identified in table 6-4, so a rigorous comparison cannot be made. However, the general observation to be made is that the products of pyrite oxidation (that is, iron, sulfate, and acidity; are present at high concentrations in some of the column test leachates, and these concentrations are of the same magnitude as those observed in actual field conditions. In addition, closer examination of tables 6-1, 6-2, and 6-3 reveals that the leachates containing the highest concentrations of pyrite oxidation products were produced primarily from refuse and coal samples. As shown in table 6-5, the refuse and coal samples collected during this study also contain the highest levels of pyrite on a percentage basis.
From the above, it appears that the column leach test employed during this study was successful in simulating the conditions necessary for the oxidation of pyrite. Furthermore, it appears that the coal waste leachates produced are very similar to leachates which would be produced under real field conditions in the absence of treatment or disposal measures implemented specifically to inhibit the oxidation of pyrite.
One final observation can be drawn from comparison of the data in tables 6-3 and 6-4: coal and coal wastes from the Western coal mining region are generally low in their pyritic sulfur content. It is notable, therefore, that the solutions produced by column leaching of Western overburden material (table 6-3) are similar in character to actual seepage from similar materials (table 6-4). Specifically, the leachates and seepage contain very low concentrations of pyrite oxidation products and trace metals, but notable concentrations of salts (as indicated by sodium). This similarity demonstrates that the column method is also capable of simulating actual leaching conditions (in this case, dissolution of salts) when pyrite oxidation is not a major factor.
Comparison of Column, EPA, and ASTM Leach Methods
The results of coal waste leaching by the column, EPA, and ASTM methods are given in Appendices E, F, and G, respectively. These results are presented in a manner to show the quantities of leached waste components in terms of (1) milligrams per liter of leachate, (2) micrograms leached per gram of coal waste, and (3) percent of waste component leached from the coal waste.
To determine whether the results produced by each of the three leach tests were significantly different, paired-t tests were utilized. The null hypothesis evaluated was that the results of the individual leach tests are the same. This hypothesis was tested for each of the waste components analyzed during this study which was detected in every leachate. In addition, visual inspection of the column leach test results revealed two distinct subsets of coal waste types: (1) coal wastes which produced acid leachates (that is, leachates containing greater than 15 milligrams per liter of acidity) and (2) those which did not. Due to the relationship of this factor to pyrite oxidation and to the design of the column leach tests, comparison of results of the column leach test to those of the other two leach methods was performed for each of these sample subsets. It was anticipated that comparison in this manner would reveal any difference in the leach methods resulting from the failure of the EPA and ASTM methods to provide the conditions necessary for the oxidation of pyrite.
The results of the leach test comparisons are presented in tables 6-6 through 6-15. Conclusions based on these results are summarized as follow:
- For the subset of samples which demonstrated an acid-generation capability by producing acid leachates during the column leach test, the column leach results were, with few exceptions, significantly different from the results of both the EPA and ASTM leach tests. (Refer to tables 6-6, 6-8, 6-10, and 6-12.) Furthermore, these results demonstrate that most of the metal parameters were leached to the greatest extent by the column method.
- For the subset of samples which did not produce acid leachates during the column leach test, the results of the three leach tests were, with few exceptions, net significantly different. (Refer to tables 6-7, 6-9. 6-11. and 6-13.) Furthermore, review of the leach test data (Appendices F and G) reveals that, generally, only very small quantities of “pollutants” were leached from this subset of samples, regardless of the leach test employed.
- On the basis of leachate concentration, the EPA and ASTM tests, with the exceptions of results for potassium and sodium, did not produce significantly different results. However, on a percent-leached basis, significant differences between these two leach methods were found for many of the heavy-metal parameters.
These conclusions reflect a clear-cut trend: the three leach tests produced similar results when compared on the basis of coal waste samples which did not generate acid leachates in the column leach test. However, these leach tests produced significantly different results when compared on the basis of those coal waste samples which did generate acid leachates during the column leach test. The reasons which account for these results are related to the design of the individual leach tests. As discussed in the previous section, the column leach test was designed to simulate actual field conditions. For this reason, the conditions necessary for the oxidation of pyrite were provided by this leach method. Specifically, the simulated rainwater was allowed to percolate freely through the waste sample and drain completely between applications to ensure the availability of oxygen and humidity within the waste sample. As discussed in Section 4, these are the conditions which are necessary for the oxidation of pyrite. The EPA and ASTM leach tests are conducted under saturated conditions, and, as a result, oxygen availability is limited. Therefore, these latter two tests do not provide for the oxidation of pyrite and, consequently, do not generate the strong acid conditions which subsequently accelerate the leaching of heavy metals contained in the waste. This is graphically demonstrated by the leach test results presented in table 6-16.
The conclusions summarized above are based on the predominant results of the leach test comparisons. As noted, however, there are a small number of exceptions to these general conclusions, discussed below.
The behavior of barium can be explained on the basis of the precipitation of this element as barium sulfate. The presence of excess sulfate in leachates from the subset of samples producing acid leachates caused the precipitation of this essentially insoluble barium salt, which resulted in low quantities of barium leached regardless of the leach test employed. Therefore, for the acid subset of samples, similar leaching results were obtained for barium by all three methods, contrary to the dissimilar results obtained for most of the other metal parameters.
Exceptions to the predominant trends were also noted for arsenic and beryllium. However, these exceptions should be regarded with caution, due to the small amount of data on which the comparisons for arsenic and beryllium were made. Arsenic and beryllium were not present above detectable levels in most of the coal waste samples collected during this study. For this reason, comparisons among leach tests for these metals were based on only two or three data pairs.
Other notable exceptions were the light metals sodium and potassium. The leaching of these metals from coal wastes is due largely to the weathering of shale and clay contained in these wastes (except in western overburdens where sodium probably results primarily from evaporite salts). However, since the weathering of these substances is differentially impacted by the acid or non-acid conditions which prevail, it is not known why two metals behave differently than the other metals analyzed during this study.
Finally, in comparison of the column and EPA leach tests, exceptions are noted for the quantities of some heavy metals leached on a percent-leached basis. This is undoubtedly accounted for by the fact that the EPA leach test is conducted under weak acidic conditions. For this reason, the EPA method simulates to an extent the acidic conditions which are generated during the column test when pyrite is oxidized. In this case, the EPA and column methods resulted in similar leaching of cadmium, nickel, and lead. It is interesting that these three metals have similar properties. It is likely that knowledge of their mineralogical associations in coal waste would further explain their leaching behavior; however, such information is not available.
The EPA method caused greater leaching of copper, manganese, and zinc than did the column method for waste samples not generating acid. It is likely that the weak acidic conditions of the EPA leach test are at least partly responsible for this result. However, further explanation would also require information about the mineralogical associations of these metals. At the present, this information is not available.
Contamination of Groundwater by Mining Waste Leachates
Scope
In this section, the potential of coal waste to contaminate groundwater by natural leaching processes is evaluated. This evaluation first considers the quality of leachates formed from coal wastes (as determined experimentally during this study) and, on this basis, identifies the types and general locations of coal wastes having the greatest potential for contamination of groundwater.
The magnitude of the potential for contamination of groundwater by coal waste leachates is evaluated by consideration of the major factors which impact on the quantity and quality of leachate produced. These factors include coal (and therefore coal waste) production trends, mineralogy of the coal wastes, the long-term leaching character of the coal wastes, and disposal practices which may enhance or diminish the rate of oxidation of pyrite contained in the wastes and percolation of water through the wastes.
Identification of Coal Wastes – Contaminant Groundwater
The column leaching experiments conducted during this study were designed to determine the quality of leachates produced from coal wastes under conditions which closely simulated actual field conditions. The results of these experiments have previously been presented in section 6 of this report. (Refer to tables 6-1, 6-2, and 6-3). These results indicate that leachates from coal preparation plant refuse and coal stockpiles can contain very high concentrations of acidity and heavy metals. Leachates from overburden samples collected during this study generally contained little, if any, acidity and very low concentrations of heavy metals. It should be noted, however, that the overburden samples collected during this study were, in most cases, mixtures of all the strata overlying the coal bed. It is generally known that individual strata of an overburden may have a net acid producing potential. One such strata had been segregated at a strip mining operation, located in West Virginia, which was visited during the study. As indicated in table 6-2, leaching of this strata (sample 28) produced a leachate much higher in acidity and heavy metal content than the other overburden leachates. It is apparent, therefore, that individual strata in some overburdens can produce acid leachates. A methodology has been developed by which net acid producing strata, if any, can be identified in advance of actual strip mining. This allows the miner to preferentially handle and dispose of the acid strata in a manner which is designed to diminish the oxidation of pyrite and, subsequently, the formation of an acid drainge.
While it appears that the bulk of coal mining overburdens are not likely to produce acid leachates, the literature does contain several reports attributing water quality degradation to runoff and seepage from mined overburden material.
In most cases, this degradation has been the result of leaching of soluble salts from the overburdens as indicated by the resulting sodium, magnesium, calcium, and sulfate ion concentrations. In the Western coal mining states where the coal and coal wastes contain much less pyritic sulfur than Eastern coals and coal wastes, it is the leaching of soluble salts which presents the major source of concern with respect to contamination of groundwater by coal mining wastes.
Therefore, preparation plant refuse and coal stockpiles represent the greatest potential sources of acid leachates containing high concentrations of heavy metals. However, it is evident that all coal mining wastes, including overburdens, have a demonstrated potential to cause significant degradation of groundwater quality. This conclusion is further supported by the data presented in table 7-1 which compares the quality of coal waste column test leachates to recommended water quality criteria for public and agricultural water uses.
Contamination of Groundwater by Coal Waste Leachates
Ultimately, the magnitude of the potential for contamination of groundwater by coal waste leachates is a function of the interaction of a number of factors which impact on leachate quality and quantity. As might be expected, the involvement of multiple factors in the leachate generation process results in a great deal of variability with respect to leachate quality and quantity on the basis of both waste type and geographic location. The remainder of this section is devoted to a discussion of the major factors which impact on the magnitude of the potential for contamination of groundwater by coal waste leachates.
Coal Production Trends
In section 3 of this report, data were presented to reflect the quantities of coal wastes which have been generated during the period of 1960-1977 by waste type and geographic location. To evaluate the magnitude of the potential for contamination of groundwater by coal waste leachates it is also necessary to know the anticipated rate of future production of coal and coal wastes.
The United States Department of Energy has published data to reflect the historical and projected rate of coal production in the United States. These data are presented in tables 7-2 and 7-3 to reflect production on the basis of coal sulfur content and mining method. These data reflect two significant future coal production projections. First, the rate of coal production is projected to increase at a rapid rate in the Western region while either increasing at a moderate rate or declining in the Eastern coal mining regions through 1995. Second, these data reflect that coal production in the West will result primarily from the mining of low sulfur coal by surface mining methods. Conversely, the production of coal in the northern Appalachian and Midwest regions will result primarily from the mining of medium to high sulfur coal by underground mining methods. The conclusion to be drawn on the basis of these projections is that, as in the past, coal preparation plant refuse resulting from the cleaning of high sulfur coal will continue to be generated primarily in the northern Appalachian and Midwest regions while overburden will be the predominant waste type generated in the western region. Therefore, the primary threat to groundwater quality resulting from coal mining wastes in the East is the generation of acid, metal laden, leachates from pyrite containing refuse while in the West the primary threat results from the leaching of soluble salts from coal mining overburdens. Given the projected increase in the rate of coal production through 1995, these threats can only be expected to increase in magnitude unless treatment and disposal practices can be implemented to effectively mitigate these threats.
Regional and Local Mineralogical Factors
On the basis of data presented in this and previous studies it is apparent that notable differences in the acid producing potential of coal mining wastes exists between regions and within localities. The much lower acid producing potential of western coals and coal wastes has already been discussed and is attributed to their generally lower sulfur content (and, therefore, pyrite content) than eastern coals and coal wastes. Regional differences have also been noted between Eastern and Western Kentucky and between Northern and Southern Appalachia with acid drainage occurring predominantly in Western Kentucky and Northern Appalachia. In general, it can be concluded on the basis of this and previous studies that the greatest incidence of acid leachate production from coal wastes occurs in the Northern Appalachian and Eastern Interior regions. However, even within these regions a great deal of variability exists between localities with respect to the acid producing character of coal wastes.
The factors which largely account for the variability noted above are regional and local mineralogical differences. Two mineralogical characteristics of coal wastes have specifically been correlated with the net acid production potential of these wastes. These characteristics are: (1) the related abundance of framboidal pyrite in a given waste and, (2) the relative abundance of minerals, such as limestone, having an acid neutralizing capacity.
Caruccio has conducted a series of studies related to the production of acid drainage from coal wastes. One finding of these studies, as discussed in section 4 of this report, is that pyrite morphology is a better predictor of acid drainage (or leachate) formation than either total sulfur or total pyritic sulfur. Framboidal pyrite was determined to be the most prolific acid producing form. Caruccio found that the other types of pyrite (that is, euhedral crystals, coarse grained masses which replaced original plant matter, and coarse grained platy masses occupying joints in the strata) decompose at a relative slow rate; the amount of acidity produced being low or easily neutralized by small amounts of alkalinity generated by associated calcium carbonate found in the wastes. On the basis Caruccio concluded that the severity of acid drainage is in part, a direct function of the amount of framboidal pyrite in coal waste exposed to the atmosphere.
Alkaline components present in coal waste have the capacity to neutralize acid which may be produced. The situation which ultimately prevails, therefore, will depend on the relative quantities of acid and alkaline components and the rate of pyrite oxidation relative to the rate of dissolution of alkaline components such as limestone. On this basis, it is apparent that the localized distribution of framboidal pyrite and limestone in strata associated with coal beds will largely determine the magnitude of the potential for production of acid leachates from mining wastes created by the disturbance or extraction of these strata.
With respect to the regional distribution of limestone, it has been observed that the limestone content of rock strata increases in north to south progression in the Appalachian region. This is undoubtedly one reason why there are few incidents of acid drainage from coal wastes in Southern Appalachia while there are numerous such incidents in Northern Appalachia.
It is also interesting to note that Caruccio and Ferm have found a relationship between the occurrance of framboidal pyrite and the paleoenvironment of the stratum based on their studies in Western Pennsylvania and Eastern Kentucky. Specifically, they found that framboidal pyrite is generally more abundant in marine-brackish water coal sequences than in fresh water coals. In addition, they noted that increases in sulfur contents of marine-brackish water coals were manifested by increases in framboidal pyrite while increases in sulfur content in fresh water coals reflect, with some exceptions, increases in massive coarse grained pyrite. In transitional zones between these paleoenvironments the occurrence and abundance of framboidal pyrite was found to be highly variable, however.
The relative abundance of framboidal pyrite and limestone in samples collected for the present study was not determined. However, review of the column test leachate results (tables 6-1, 6-2, and 6-3) reflect that some of the samples with relatively high pyrite contents (table 6-5) produced highly acid leachates while others (notably samples 13 and 14) did not. This observation is in agreement with the findings of Caruccio since it is obvious that the total pyrite content alone did not determine the character of leachates produced during this study. In this regard, it is notable that the pyrite present in samples 13 and 14 was visibly detectable and embedded in small shell fossils that were abundantly present on the surfaces of shale contained in these waste samples. It is unlikely, therefore, that much of the pyrite in these samples was framboidal pyrite.
Long-Term Leaching Character of Coal Wastes
Information and data to describe the long-term leaching character of coal mining wastes is extremely limited or lacking. In addition, no previous studies have investigated the long-term leaching characteristics of heavy metals. Therefore, during the present study it was desired to investigate the rate at which coal wastes are leached over a long period of time. This was accomplished by leaching coal wastes collected during this study by a column leach method specifically designed for the purposes of this study. As previously described, this column leach test was conducted for a period of 5 months and closely simulated actual field conditions to which coal wastes are exposed in waste disposal areas.
The chemical characteristics of the coal waste leachates produced and the rates at which these components were leached from the wastes have been summarized in tables 7-1 and 7-4. These results reflect distinct differences between coal wastes producing acid leachates (that is, greater than 15 milligrams per liter of acidity) and those which do not.
Heavy metals present in the non-acid producing overburdens and preparation plant refuse leach at a very slow rate, as evidenced by the low percentage of metals leached from these wastes during the 5-month period of the column leach test (table 7-4). As a result, the concentration of heavy metals in leachates from these wastes are also low and, as shown in table 7-1, are within the limits of recommended water quality standards. Over the long-term this situation would not be expected to change. Therefore, coal wastes which do not produce acid leachates have little, if any, potential to contaminate groundwater by leaching of acid or heavy metal components. However, these wastes do contain leachable salts which do have a potential to degrade groundwater quality. As indicated in table 7-1, sulfate ion concentrations in leachates from these wastes are often more than a magnitude greater than recommended water quality supplies. In addition, for all public water supply and agricultural uses sulfate and sodium ion concentrations less than 200 milligrams per liter have been recommended. Therefore, it is apparent that the leaching of soluble salts from non-acid producing coal wastes represents the primary threat of these wastes to groundwater quality.
In contrast to the non-acid producing wastes, the heavy metal concentrations in leachates from acid producing coal wastes exceed recommended water quality standards by several orders of magnitude in most cases. The data presented in table 7-4 also reflect that metals in these wastes are Leached at a very fast rate, although the rates do vary over a broad range reflecting differences between specific metals and waste samples. The stockpiled coal samples leached during this study behaved similar to the acid producing coal wastes. Therefore, it is evident that given waste piles or coal stockpiles which produce acid leachates represent a significant threat to groundwater quality.
Coal stockpiles are created and depleted in response to prevailing market conditions and therefore their existence is transitional. Furthermore, it is rare that a given stockpile will remain at a mine site for an extended length of time. For this reason, long-term (that is, greater than 1 year) leaching of such piles would not usually occur. Conversely, however, the disposal of preparation plant refuse and overburdens is usually permanent and, therefore, the long-term leaching characteristics of these wastes are of major concern. On the basis of the data presented in table 7-4, a number of observations can be made in this regard.
A number of studies of coal waste piles have provided evidence that a tone of weathering exist in the top 6—15 inches of these piles and that below this zone little or no oxidation of pyrite or leaching of metals occurs. To simulate this condition, coal waste samples collected during this study were loaded into the columns to a depth of 15 inches. As discussed in section 5 of this report, the column leach tests were also conducted in a manner to simulate one annualized rainfall cycle for the geographic locations in which the wastes were collected. Therefore, it is assumed that the results of the column leach tests simulate the magnitude of leaching which occurs in coal refuse piles or spoil banks during one year subsequent to disposal of these wastes.
The results presented in table 7-4 indicate a major percentage of the metals present in the acid producing refuse and overburden were leached during the course of the column leach tests. For most of the metals analyzed, as much as 10 to 70 percent of the leachable metal content was leached. In some instances, 100 percent of the leachable metal present was leached. A large percentage, 2 to 60 percent, of the iron present in these waste was also leached which indicates that pyrite oxidation also proceeded at a fast rate. On this basis, it can be concluded that over a period of approximately 3 to 10 years the reactive pyrite and leachable metal present in the surface of an inactive refuse pile or spoil bank would totally be removed by leaching. However, if significant erosion of the surface of the refuse pile or spoil bank occurred, this situation could change dramatically as fresh material would be exposed to oxidation and leaching. Therefore, the long-term potential of an inactive refuse pile or spoil bank to contaminate groundwater by leaching of acid and metals will be a function of the rate of leaching (that is, depletion) relative to the rate of erosion of the surface of the pile (that is, “renewal” of unleached waste). In the case of an active refuse pile, the continuous disposal of fresh material on the pile would provide the “renewal” process and sustain the waste potential for generation of acid, metal laden leachates.
Importance of Regional Rainfall Amounts
The percolation of rainwater through coal wastes serves as the transport media for removal of pyrite oxidation products, solubilized metals, and soluble salts from these wastes. Therefore, the importance of rainfall is related to its role in transporting potential contaminants from their source of origin.
The regional precipitation volume which falls annually is undoubtedly one of the factors which determine the volume of leachate which ultimately emanates from a given source. However, this factor is not necessarily related to the quantity of acid, metals or salts leached from mining wastes except in arid regions where rainfall volume may be a limiting factor. As previously discussed in section 4, it has been observed that the quantity of contaminants which can be generated and flushed from a coal stockpile or refuse pile is dependent upon the amount of reaction time allowed to occur within the coal pile or refuse before the contaminants are flushed from the pile by precipitation. Therefore, it is apparent that the quantity of contaminants leached is a function of the rate processes of chemical oxidation and diffusion controlled dissolution of salts. These rate processes are not directly a function of the volume of water percolating through the wastes and, therefore, the quantity of contaminants leached is not a direct function of regional rainfall quantities.
It has previously been noted that very little actual leaching of coal overburdens may occur in the western coal mining region due to the low volume of precipitation and high rates of evaporation that occur over most of the region. Therefore, rainfall volume appears to be a limiting factor in the western coal mining region and, as a result, the potential for contamination of groundwater due to leaching of coal wastes by rainwater in this region may be minimal. As shown in figure 7-1, however, the annual average precipitation rate exceeds 30 inches over the entire eastern portion of the continent and, therefore, is not a limiting factor in this area.
During the present study, the volume of simulated rainfall used for leaching of coal wastes by the column method was determined on the basis of the average annual precipitation in the geographic location from which the coal waste was collected. As expected on the basis of the above discussion, no correlation was found between the quantity of acid or metals leached and the annual precipitation rate.
Waste Disposal Practices
One of the conclusions of a recent study of waste disposal practices and their effects on groundwater is that proper site selection as well as proper operation and maintenance of facilities, is the principal technique available for minimizing groundwater contamination problems. Throughout the present report, the importance of the pyrite oxidation process and its determinative impact on the quality of leachate which results from mining wastes has been stressed. It is evident, therefore, that disposal practices which effectively ameliorate the pyrite oxidation process, either by slowing the rate of this process or by minimizing the contact of pyrite containing wastes with percolating water, will reduce the groundwater contamination potential.
As a result of the Surface Mining Control and Reclamation Act of 1977, a number of disposal practices specifically designed to ameliorate the oxidation of pyrite and flushing of disposed wastes have evolved. These include segregation and subsequent burial of acid spoils during strip mining and reclamation of preparation plant refuse disposal areas. This latter process usually includes covering the refuse with a soil cover, implementation of measures to prevent erosion, and the use of drains to minimize the contact of water with the wastes. It must be noted, however, that these practices were not used extensively prior to the Reclamation Act and long-term data to evaluate their effectiveness is generally not available at the present. However, a few studies to acquire this information are on-going.
Recommendations
- This and previous studies demonstrate conclusively that coal mining wastes have a very great potential to contaminate groundwater as evidenced by the quality of leachates produced from these wastes. However, very few studies have been performed to investigate the quantity of leachates formed from these wastes. Such studies are needed before the potential of mining wastes to contaminate groundwater can be fully evaluated. These studies should especially consider the extent to which various waste disposal methods minimize the quantity of leachate formed.
- The EPA extraction procedure or the proposed ASTM shake test should not be used for evaluation of the teachability of pyritic mining wastes. These tests do not replicate the impact of the pyrite oxidation process and, as a result, fail to accurately predict the extent to which pyritic mining wastes generate acid. A secondary consequence is the failure of these tests to accurately predict the extent to which heavy metals are leached from these wastes. Therefore, additional developmental work is required to design a short-term test that will accurately predict the leachability of mining wastes.
- With the passage of the Federal Surface Mining Reclamation Act, in addition to various state regulations governing mining waste disposal, a number of waste disposal techniques have evolved which are designed to ameloriate the formation of acid drainage from mining wastes. These techniques include burial of ‘toxic’ wastes, compaction and sealing of refuse piles with clay or soil, revegatation of piles, etc. While the short-term effectiveness of such practices have been demonstrated in some instances, their long-term effectiveness is not known. Therefore, long-term studies are needed to provide the data required to fully evaluate the effectiveness of these disposal methods and to identify the methods which are most effective in preventing contamination of water resources.
- To evaluate existing mining waste disposal methods or to develop new, more effective methods it is necessary to understand the relative importance of the factors that determine the quality and quantity of leachate which emanates from the disposal area. As indicated during this study a number of physical, chemical, and biological factors are involved. It would be useful, therefore, to quantitatively determine the relative impact of these factors. A model could then be developed to predict the quantity and quality of leachate which could be expected from a given mining waste when the disposal conditions were known. This information would be useful for defining optimum disposal methods or remedial measures.
- Leachate generated from stockpiled coal was determined during this study to be highly contaminated with acidity, metals, and sulfate ion. Therefore, a significant potential for contamination of groundwater and surface water from this source exists and can be expected to expand as reliance upon coal as a fuel source increases. The significance of this source is that it is not limited to coal mining and preparation sites but is more widespread including shipping terminals, transfer points, storage yards, power generating stations, coking operations, and any other site where stockpiled coal is exposed to the atmosphere. The proximity of many of these sites to populated areas also provides the potential for contamination of major water supply aquifers. It is recommended therefore, that this situation be further evaluated.
- Some researchers have found that under laboratory conditions certain bacteria can greatly increase the rate of oxidation of pyrite in coal wastes. However, other researchers are skeptical that real field environmental conditions would favor the establishment of a population of these bacteria in coal wastes that would be sufficient in size to have a significant impact on the rate of pyrite oxidation. In view of this controversy, further definitive research is required to determine the quantitative impact that iron oxidizing bacteria have on the rate of pyrite oxidation under actual field conditions.
