Table of Contents
This report describes possible material application as oxygen electrode catalysts. The ultimate objective was to assess the potential of abundant, less costly materials as substitutes for platinum, or to increase the efficiency of platinum when used as an oxygen electrocatalyst. Presently, imports account for essentially all of the U.S. requirement for primary platinum metals.
The development of practical fuel cells for large-scale electrical power generation could have a significant effect on the Nation’s energy situation. Because the fuel cell is an isothermal device, it is not subject to the efficiency limitations of the Carnot cycle principle, as are conventional steam-generating processes.
The major problem hindering the realization of practical fuel cells lies at the oxygen (or air) electrode. The oxygen molecule is kinetically very stable, and for oxygen reduction to take place, an active catalyst is necessary to facilitate electron transfer to the cathode. Platinum, which is presently the most active known oxygen electrocatalyst, produces oxygen reduction rates on the order of 100 times less than the rate of electro-oxidation of hydrogen.
If a fuel cell is to be operated with a carbon-containing fuel, the use of an acid electrolyte is desirable because it easily rejects the carbon dioxide produced. This places an additional demand on the oxygen electro-catalyst and, hence, platinum. The catalyst must possess chemical stability approaching that of platinum to withstand the extremely corrosive conditions of high oxidation potential in acid electrolyte.
Most of the recent studies of materials other than platinum for oxygen electrocatalysts have dealt with binary and ternary oxides with known acid stability. Considerable attention was directed toward the mixed oxides known as tungsten bronzes. These compounds have the composition AxWO3, where A is alkali, alkaline earth, lanthanide, or actinide metal, and x may range from zero to 1. Early work reported high activity for the bronzes; some were claimed to be nearly as active as platinum. Subsequent investigation, however, showed that the high activity was due to a platinum impurity introduced during preparation of the tungsten bronze. The bronzes have not yet been shown to be practical fuel cell catalysts.
The National Bureau of Standards prepared a number of mixed transition metal oxides with known or expected stability toward acids and evaluated their electrochemical behavior. This study suggested promising materials for further work, but it did not conclusively show that any of the oxides were sufficiently active for use in a fuel cell.
A large number of interstitial compounds of Fe, Co, and Ni were prepared by Akhtar, Grein, and Bienstock. They were evaluated as fuel cell catalysts in several different electrolytes; however, none were found to be especially promising for oxygen reduction in acid electrolytes.
The catalytic activity of tungsten carbide (WC) has received considerable attention in recent years, since it was suggested by Levy and Boudart that WC is much like platinum in its catalytic activity. Additional work on the electronic structure of WC supports this point of view.
Materials were selected for the present study mainly on the basis of their good electrical conductivity, their known resistance to acids, and/or the fact that they contain transition metals whose D-type electrons result in considerable catalytic activity for other reactions. The main part of this investigation involved a screening process to eliminate the majority of materials which could be predicted as unsuitable. Additional work was done on promising materials in an attempt to improve their activity with inexpensive modifications or to use the materials to improve the efficiency of platinum utilization.
Material Preparation
The purity of the catalyst material can be of great importance. If impurities react with the electrolyte, a false indication may be given concerning the acid resistance and catalytic activity of the material.
The appendix table lists the purities of commercially available materials that were examined. Most of the simple compounds, such as the carbides, silicides, chalcogenides, borides, and nitrides, were purchased from commercial firms, and had quoted purities of 99.9 pct.
More complex materials were made by methods described in the literature, but the degree of success in producing these materials in pure form varied considerably (table A-1).
For the materials reported herein, a considerable effort was made to eliminate the effects of impurities that could dominate the results. For example, many of the prepared samples were leached in electrolyte for a few days prior to testing, and atomic absorption was used to check the leachant for traces of dissolved metals. Where applied, this provided a sensitive test for acid stability that may be of value for other applications. For the samples produced by sputtering and carburization, X-ray diffraction analyses were very insensitive due to the small amounts of sample and, amorphous nature of the film produced.
Since the main objective of this investigation was to screen catalysts and not to perfect preparation techniques, no attempt was made to improve the quality of the materials beyond minimum requirements. Many other materials were considered and abandoned simply because of problems associated with upgrading their purity.
Electrode Preparation
Most of the electrodes were fabricated by making a powder of the material to be examined and then mixing the powdered sample with acetone and spreading the slurry evenly over both sides of a 0.65- by 2.5-cm gold foil. After the acetone evaporated, the powder was pressed into the gold foil by sandwiching the powder-covered gold foil between two sheets of tantalum and passing the sandwich through a small rolling mill. This technique worked well for the majority of the samples, but, in special cases, other techniques were used.
For example, several samples were prepared by sputtering small but varying amounts of platinum onto different substrates. The objective was to determine if the sputtering technique could result in more efficient use of platinum and whether WC made a substrate superior to the gold. The sputtering was carried out in a modified vacuum evaporator, which was pumped to about 5 x 10 -6 torr before admitting high-purity argon gas. One series of samples consisted of platinum sputtered from a 2-in-diam cathode for 15 sec, 40 sec, and 120 sec.
The argon pressure was 30 millitorr (mt), the sputtering voltage was 2 kv, and the current was 5ma. Another series (Pt-1 and Pt-2) consisted of sputtering platinum onto a gold foil and, simultaneously, onto WC that was imbedded into a gold foil. In this case, the voltage was reduced to 1 kv, the pressure reduced to 20 mt, and a current of only a few milliamps resulted. Sputtering time ranged from 10 to 250 sec, and a separate calibration showed a deposition rate of 5±2 A/min.
Test Cell Construction and Operation
Electrode materials were tested in an all-glass electrochemical cell (fig. 1) operated galvanostatically with a platinum-wire counter electrode. The 1N H2SO4 electrolyte was prepared from reagent-grade sulfuric acid and double distilled water; the second distillation was conducted in a glass still. Electrode potentials were measured relative to a dynamic hydrogen electrode with Luggin capillary in the same electrolyte. This reference electrode, similar to that described by Giner, was found to be stable for more than
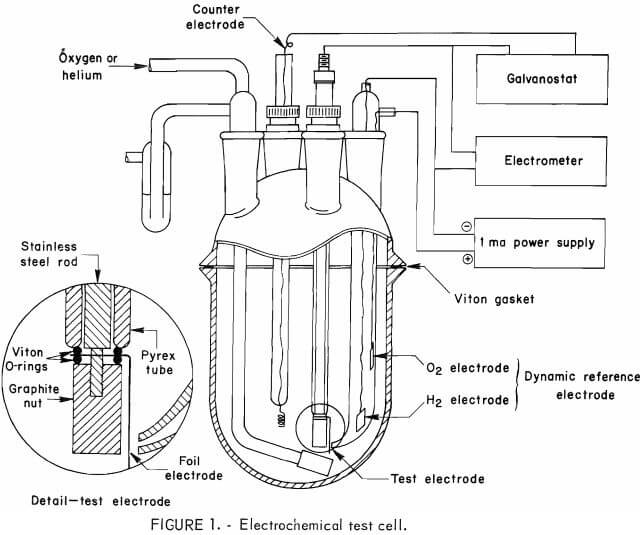
6 months. Cell current was supplied by a potentiostat-galvanostat; the potential was measured by a chart recorder through an electrometer amplifier with an input resistance of 10 12 ohms.
 Oxygen (99.99 pct) or helium (99.999 pct) supplied through a glass frit directly under the test electrode provided agitation. All tests were made at ambient temperature and pressure (approximately 23° C and 650 mm Hg).
Oxygen (99.99 pct) or helium (99.999 pct) supplied through a glass frit directly under the test electrode provided agitation. All tests were made at ambient temperature and pressure (approximately 23° C and 650 mm Hg).
Each electrode was pretreated in the oxygen-saturated electrolyte by applying an anodic current of 10 -3 amp for a few minutes before beginning the cathodic test sequence. The pretreatment served to uniformly oxidize the surface of each electrode and to increase the rate at which the electrode reached an open-circuit rest potential.
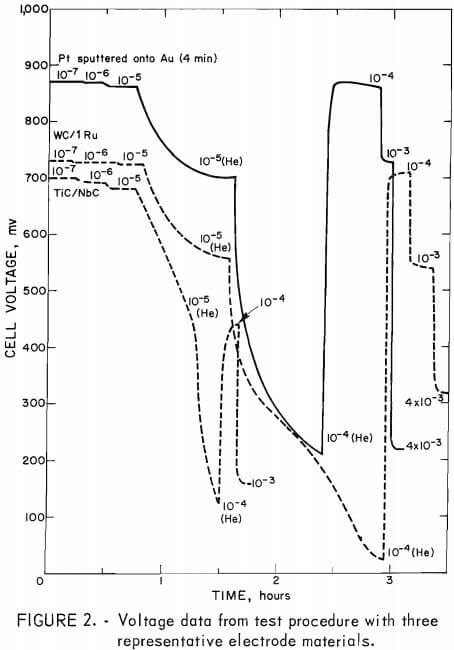
A typical test began by measuring the open-circuit voltage under oxygen gas. The cathodic current was increased by factors of 10, from 10 -7 to about 10 -3 amp, and the cell voltage was monitored with the chart recorder. After the voltage reached a steady-state value at each step, the current was increased to the next value. After the 10 -5 amp test, the oxygen was replaced by helium to evaluate the influence of any reactions not associated with oxygen reduction. The oxygen was returned after the voltage was measured under helium at 10 -5 and 10 -4 amp. Figure 2 shows the test results for the following representative samples: platinum sputtered for 4 min onto the gold foil electrode, WC with 1 at. pct ruthenium prepared by carburizing WO3 with ruthenium, and TiC-CbC solid solution.
Results and Discussion
Three sequential procedures were used for screening the candidate materials: (1) stability in 1N H2SO4 (2) stability in 1N H2SO4 with an applied potential, and (3) measurements of voltage versus current produced when oxygen was bubbled around the cathode, that is, catalytic activity. A summary of the results is presented in table A-1. In most cases, the first test consisted of placing the sample in the 1N H2SO4 acid and observing obvious changes in the material. In a few cases, the acid was analyzed for traces of components of the material being tested. Any material that failed at this point was not investigated further in the subsequent time-consuming tests. Some materials that passed the acid solubility test demonstrated a lack of stability in the test cell by producing a voltage that was about the same with both oxygen and helium flowing over the cathode. In these cases, a reaction is denoted in table A-1. Observations showed that at small currents such as 10 -5 amp some voltage was produced with a helium flow, even with platinum electrodes; it was assumed to be due to unavoidable impurities in the electrolyte and/or sample material. At higher currents the voltage with a helium flow dropped to some value less than 100 mv for the stable, high-purity materials.
Each catalytic activity test produced a large amount of data (fig. 2). Therefore, for purposes of comparison, table A-1 shows the measured voltage at a current density of 10 -4 amp per 3.2 cm² of geometrical area. (No attempt was made to measure the true surface area of the electrodes.) This voltage represents a good compromise since the spread observed in peak voltages is higher than it would be at larger currents, yet the current is high enough to prevent the voltage from being strongly influenced by the small unavoidable concentration of impurities mentioned earlier. Although the theoretical equilibrium potential of 1,200 mv was never achieved at open circuit, the open-circuit voltage for the platinized platinum standard, 1,050 mv, was typical of that obtained in other test cells of the type used here. No attempt was made to achieve the theoretical voltage for the reaction, and the open-circuit voltages are not quoted because of their dependence on small concentrations of impurities. The voltages in table A-1 are quoted to the nearest 10 mv; however, the overall reproducibility of the data, including variations in sample and electrode preparations, is probably ±50 mv.
The uncoated gold foil substrate produced 520 mv at 10 -4 amp. However, this activity should not be considered a “blank” for the other tests. Since both the coating-and-rolling and the sputtering procedures covered essentially all the gold surface, very little contribution to the measured activity would be expected from the gold substrate. The very low voltages measured for compounds such as Ni3Ti (zero mv) showed that the gold foil served as an essentially inert substrate.
Conclusions for the different classes of materials follow: Most of the tested carbides, silicides, and phosphides produced low voltages indicating little if any catalytic activity. Many of the borides, chalcogenides, and nitrides reacted, and those that did not react produced low voltages. One of the carbides (WC), which has been examined as a catalyst for other reactions, appeared to be promising and was chosen for further studies to be discussed below.
Several solid, solutions of carbides, nitrides, and carbonitrides were examined with some improvements realized. For example, three of the 50 at. pct binary solid solutions (TaC-ZrC, CbC-ZrC, and TiC-ZrC) had significantly higher activity than either of their constituents. Solutions of TaC-CbC and TiC-CbC were about the same, but TiC-TaC was lower in activity than either constituent. As an additional check, TaC-CbC and TaC-ZrC were prepared but were not arc melted. They produced activities about the same as expected from the activities of the constituents. Although the improvements in activity of the solid solutions are interesting and suggest possibilities for further work, the magnitude of the improvement was not large enough to warrant further work in the screening studies reported here.
No similar improvements were observed for solid solutions of nitrides or carbonitrides, although it is interesting to note that the (TiN) 0.5 (ZrN) 0.5 and the (TiN) 0.5 (CbN) 0.5 were much more stable in the 1N H2SO4 acid than the TiN or ZrN separately. Mixing TiC, ZrC, or CbC with TiN also produced a more stable solid solution than the pure TiN, but no improvement in activity was observed.
None of the mixed oxides that were examined produced very high voltages although many of them were stable in the conditions of the oxygen electrode.
A few pure metals and intermetallic compounds were examined. As expected, the pure platinum and ruthenium were quite active, and gold was considerably less active. Low activity, coupled with acid resistance and high ductility, was the reason gold made a suitable substrate for test electrodes. Of the intermetallics prepared, only TiRu exhibited significant electrocatalytic activity and may be deserving of further work.
A series of experiments was made to determine whether the activity of platinum electrodes might be enhanced by applying the platinum by sputtering. Also of interest was the effect of the substrate on the activity of sputtered platinum. When either gold foil or WC was sputtered with platinum, the electrocatalytic activity was found to increase to a maximum, as coating thickness was increased to about 20 A. Beyond that thickness, activity was unchanged. Activity tended to be higher for the gold substrate electrodes than for the WC substrate. In any case, the sputtered electrodes did not exhibit significantly greater activity than other platinum samples.
Several commercially prepared catalysts (Graphimet) having platinum-group metals intercalated in the graphite lattice were tested and found to be very active. Unfortunately, it is difficult to compare the various metals in Graphimet with the same metals in our doped carbide samples because of the very high surface area of the Graphimets versus the low surface areas of the doped carbide samples.
The previous work with WC suggest its use as an oxygen electro-catalyst. In the present investigation, WC was found to be most active of the carbides tested, but not nearly as active as platinum. Various modifications then were made to WC in attempts to improve catalytic activity. It should be recognized that W2C, which is often an impurity in WC, is not very active; however, it is stable at the oxygen electrode so that small amounts of W2C impurities represent no significant problem.
Two WC samples were damaged by radiation; one with a proton beam, the other with gamma rays. No significant change in activity was observed when these were compared with the as-received WC.
A first attempt at doping consisted of firing the WC at 1,400° C with ruthenium powder mixed into the WC and into pure carbon. The ruthenium, in concentrations of 0.1, 1, and 3 at. pct did not produce higher activity than the pure WC. The sample of pure carbon with 1 at. pct of ruthenium did not perform nearly as well as WC with 1 at. pct of ruthenium.
Ross and Stonehart reported that WC, which is deficient in carbon, is more active as a catalyst. Savata and Zabronsky showed that the presence of chloride during carburization of tungsten greatly increases the number of carbon vacancies in the WC lattice. Furthermore, it has been shown that by adding small amounts of certain metals, the effect of chloride can be moderated or increased. For example, the addition of nickel almost completely eliminated chloride-induced defects, but 1 at. pct copper with chloride resulted in a highly defective structure.
To evaluate the effects of these modifications on the electrocatalytic activity of WC, a modified WC was prepared using a procedure similar to that of Savata and Zabronsky. Tungsten oxide (WO3) was mixed with a solution of either ammonium chloride or a metal chloride to make a paste with the composition shown in table A-1. The paste was heated at 850° C for 10 hours in a stream of 350 cm³/min H2 plus 6 cm³/min CH4 to give complete conversion to WC.
No significant improvement in catalytic activity was observed for the WC preparations modified with NH4Cl, CuCl, or NiCl2. When additions of platinum-group chlorides were made, activity was increased substantially; however, the increase was apparently due only to the activity of the platinum metal rather than the effect of any chloride-induced defects. The direct addition of elemental ruthenium to WC produced the same activity as did the ruthenium-chloride-modified carburization technique.
Summary
Transition metal silicides, phosphides, borides, chalcogenides, oxides, nitrides, and most of the carbides either gave low catalytic activity or reacted with the electrolyte. Some increases in activity were produced with solid solutions of carbides and by doping carbides with platinum-group metals.
The conclusions from this work fall into two categories: fundamental and applied. When trying to unravel the extremely complex nature of catalyst behavior, it is important to recognize such facts as the improved activity of (TaC)0.5(ZrC)0.5 compared with either TaC or ZrC and the relative activities of the various transition metal carbides, silicides, etc. Even the differences observed in activity between platinum sputtered onto gold and platinum sputtered onto WC are important for a fundamental understanding of electrode catalysts. However, no practical replacement for the expensive platinum-group metals was found, nor was a successful scheme devised for making more efficient use of these metals.
The data presented should be of assistance to catalyst researchers and may speed the development of an efficient catalyst material suitable for use as a substitute for platinum in such applications as fuel cells.
Many materials including carbides, silicides, phosphides, borides, nitrides, oxides, and metals were studied by the Bureau of Mines as potential fuel cell catalysts for electroreduction of oxygen in a 1N H2SO4 electrolyte. The objective of this study was to assess the potential of abundant, low-cost materials as substitutes for platinum or to increase the catalytic efficiency of platinum. Several compounds were rejected because of their reaction in the corrosive environment of the oxygen electrode. However, some of the remaining were the most active catalysts.
Activity of carbides such as tungsten carbide could be improved by doping with platinum-group metals, by varying stoichiometry, or by sputtering on a layer of platinum. While no catalysts were found with activity as high as platinum, some compounds were shown to be deserving of further investigation.
