Objective
Provide an environmentally compatible means of producing lead from galena concentrates that replaces the current high-temperature pyrometallurgical primary lead smelting process and supplies pure lead metal within present and proposed Environmental Protection Agency (EPA) and Occupational Safety and Health Administration (OSHA) lead emission standards.
Approach
The U.S. Bureau of Mines developed an energy efficient and pollution-free leach-electrowinning method for use in recovering pure lead metal and byproducts from lead sulfide concentrates.
How It Works
The present method for smelting lead sulfide concentrates to produce lead is a pyrometallurgical smelting method that requires temperatures as high as 1,400 °C. Such smelting methods result in high lead and sulfur dioxide emissions. These high-temperature smelting operations will be unable to meet proposed emission standards. Unless new technology is developed or some compromise is reached, such operations could be forced to close. The U.S. Bureau of Mines developed a lead recovery process that promises to provide a pollution-free alternative to high-emission smelting methods. Using the Bureau’s new process, galena concentrates are leached directly under oxidative conditions in waste fluosilicic acid at tem-
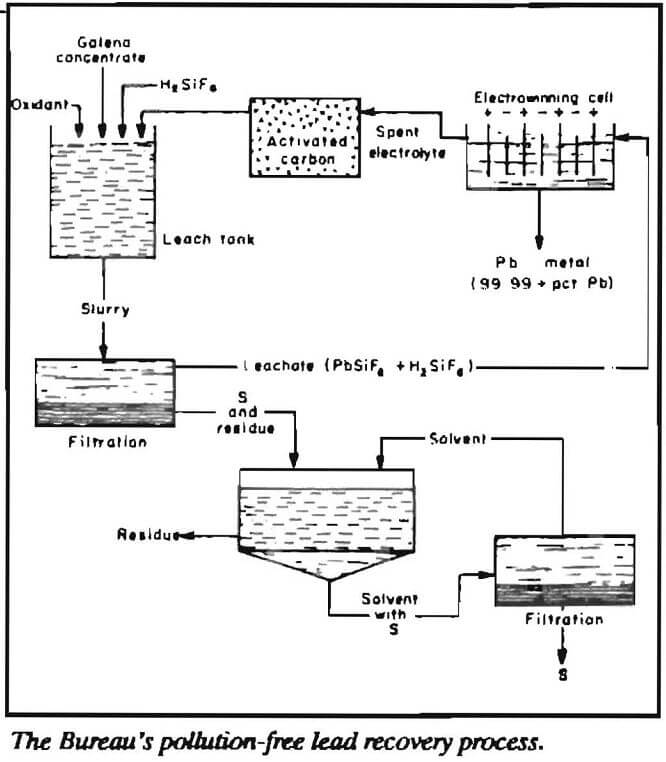
peratures less than 120°C. This solubilizes the lead and produces a residue containing elemental sulfur and other metal values. Pure lead metal (99.99 pct) is recovered from the leach solution by electrowinning using inert anodes and lead cathode starting sheets. To prevent lead dioxide formation at the anodes during the electrowinning step, phosphorus is added to the fluosilicic acid electrolyte. The amount of energy consumed during the entire process is an efficient 800 kilowatt hours per ton of lead produced.
The new method produces lead metal pure enough for any application; conventional smelting methods produce a pure lead product only after extensive refining to remove antimony and other impurities. The refining method used by smelting operations can result in further lead emission problems.
Another advantage of the Bureau’s recovery process is that the leach-electrowinning method converts sulfur in the lead sulfide concentrates to elemental sulfur. Existing smelting methods produce sulfur dioxide, some of which is captured and converted to sulfuric acid. Of the two conversion products, elemental sulfur is more desirable because it is of more value per ton and is the best form for shipping at the greatest value. Sulfuric acid is three times as heavy as sulfur. In addition, the leach residue of the Bureau’s process contains silver, zinc, copper, and cobalt, making it a valuable byproduct.
Test Results
Successful 1-liter scale bench tests were followed by leaching in a 100-liter tank. Lead extraction was 96 pct when galena concentrates were leached in recycled waste fluosilicic acid using hydrogen peroxide and lead dioxide as oxidants. After solid-liquid separation to obtain the lead-fluosilicate solution and the residue containing elemental sulfur, pure lead metal was electrowon in a 20-liter cell containing four titanium anodes coated with lead dioxide and three pure lead cathodes. Approximately 99 pct of the elemental sulfur was separated from the leach residue with tetrachloroethylene and kerosene at 115°C.
The fixed capital cost of a plant designed to recover 300 tons of lead per day has been estimated to be about $36 million, based on third- quarter 1985 equipment costs. Net operating costs are estimated to be about $0.22 per pound of lead. With the August 1988 market price of lead at $0.36 per pound, the potential return on investment of $0.14 per pound makes this process economically attractive.
Patent Status
The U.S. Department of the Interior does hold several patents on this process. These include U.S. patents 4,159,231, 4,272,340, and 4,500,398. For additional information about these patents or on patent licensing, please contact the Bureau of Mines, Office of Technology Transfer, M.S. 6201, 2401 E Street, NW, Washington, DC 20241.
For More Information
Additional information about this process can be obtained from U.S. Bureau of Mines Report of Investigations (RI) 9055, “Hydrometallurgical Process for Producing Lead and Elemental Sulfur From Galena Concentrates.”
