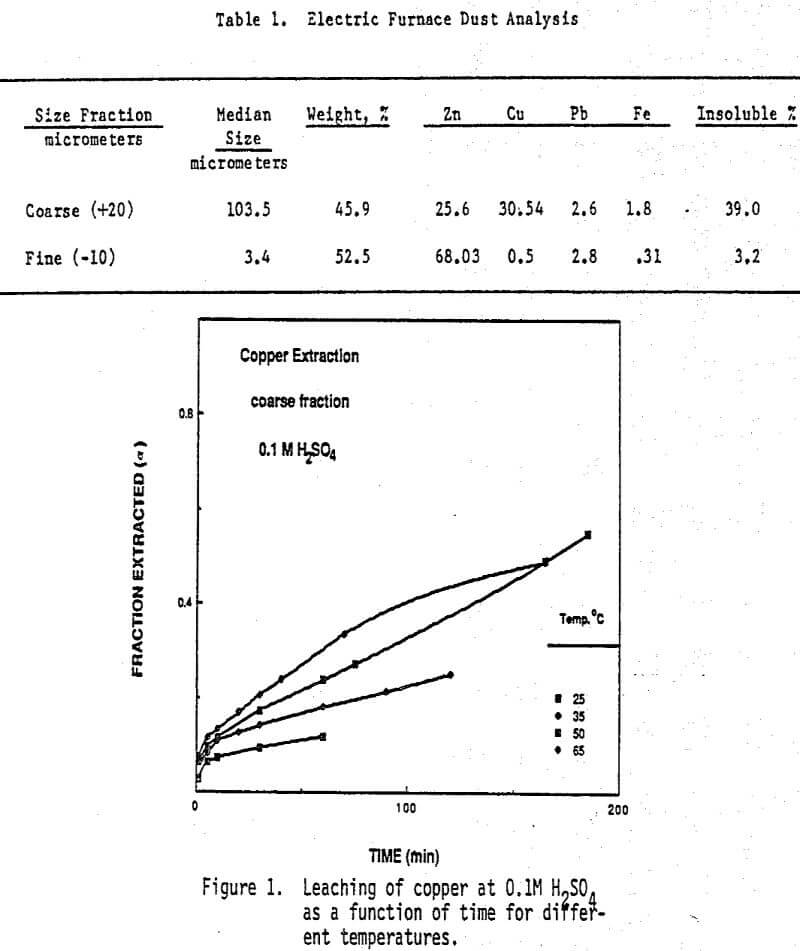
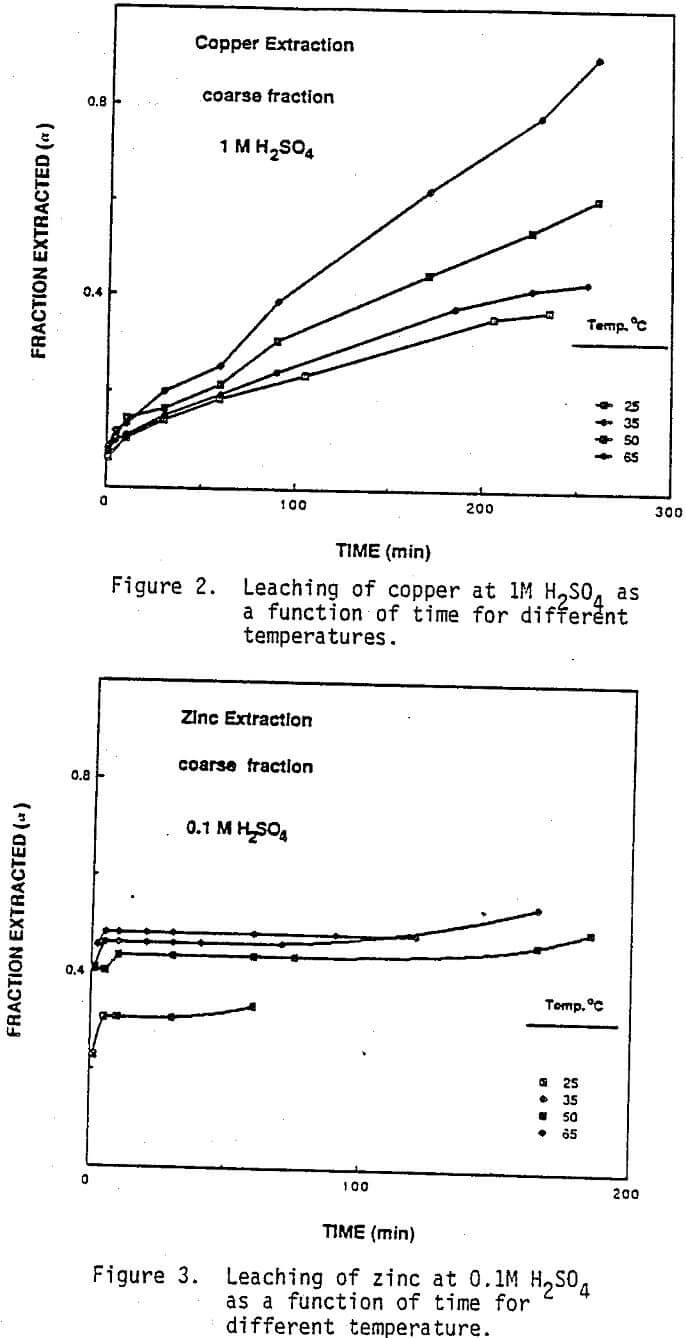
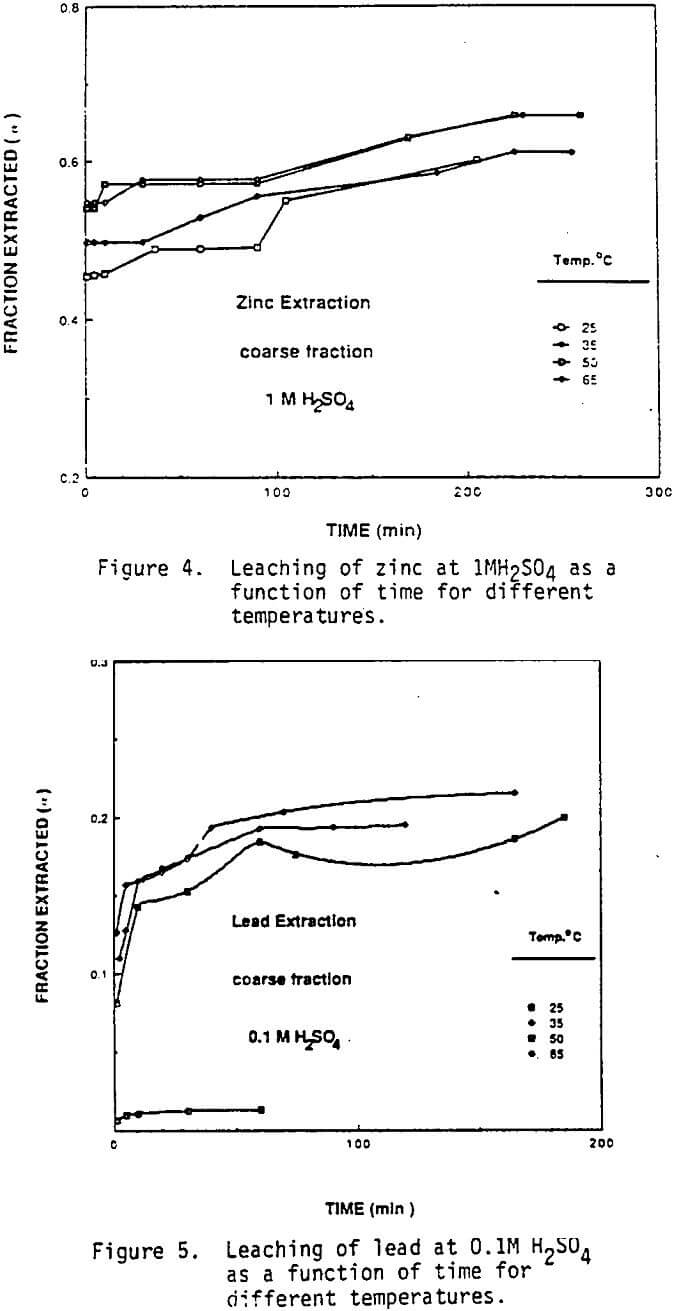
Effect of Temperature and Acid Concentration: The effect of temperature on the leaching behavior of copper and zinc is shown by the data in Figures 1 through 4. In these experiments, leaching solutions were well stirred at 550 rpm and open to air. “Fraction Extracted” refer to that portion of the total copper and zinc that has been dissolved at time t. The data for copper extraction given in Figures 1 and 2 show that compared with zinc, the rate of copper extraction at least initially was somewhat slower than the rate of zinc extraction as shown in Figures 3 and 4. In addition, temperature had a more significant effect on the rate of copper extraction than on zinc extraction. Acid concentration, on the other hand, had very little effect on the rate of copper dissolution. For example, in 0.1M sulfuric acid- 50% of the copper was solubilized in 165 minutes at 65 C, (Figure 1). When the acid concentration was increased to 1M, Figure 2, the level of copper extraction for the same time and temperature was practically identical. Note that kinetics of copper dissolution at both acid concentrations were less than 10% at 10 minutes leaching time. However, higher temperatures increased the rate of copper extraction.
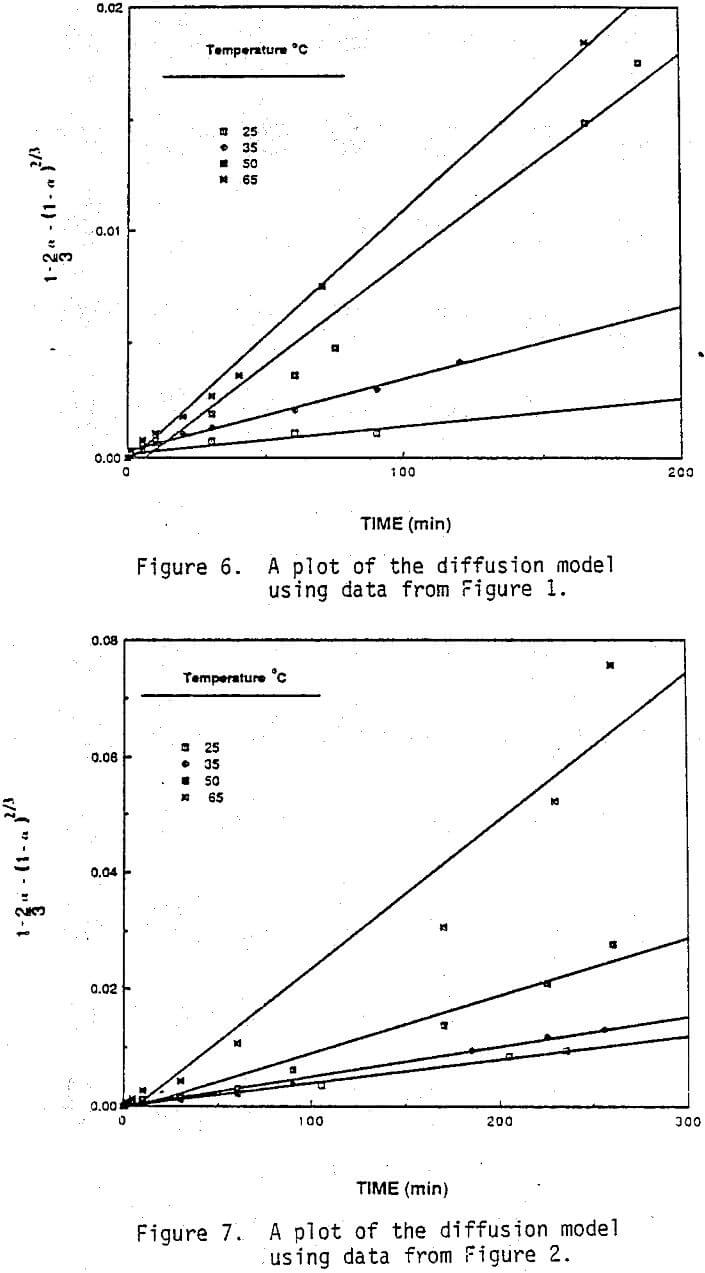
The fraction of zinc extracted as a function of temperature for two acid concentrations is given in Figure 3 and 4. Although the rate of zinc extraction was rapid in both 0.1M and 1.0M H2SO4 it was clearly more rapid in the more acidic solution. About 50% of the zinc can be extracted within five minutes for nearly all conditions. Temperature had a relatively mild effect on the rate of zinc extraction. The initially rapid rate of zinc dissolution and its dependence on acid concentration suggest that much of the zinc, is present in the dust as zinc oxide and that the balance is present in a less soluble form such as metal alloy or perhaps ferrites.
The fraction of lead extracted as a function of temperature is given in Figure 5. It is evident that at 25°C, the amount of lead extracted from the flue dust is minimal. An increase in temperature increased the extraction up to 0.2. Unlike the results observed for zinc, higher acid concentration had no significant influence on the leaching kinetics of lead. These observations are consistent with the limited solubility of lead sulfate in these solutions. (Linke, 1965) Further investigations on the leaching kinetics of lead from this material as well as other types of waste (e.g. foundry sand) are underway and will be reported in subsequent publications.
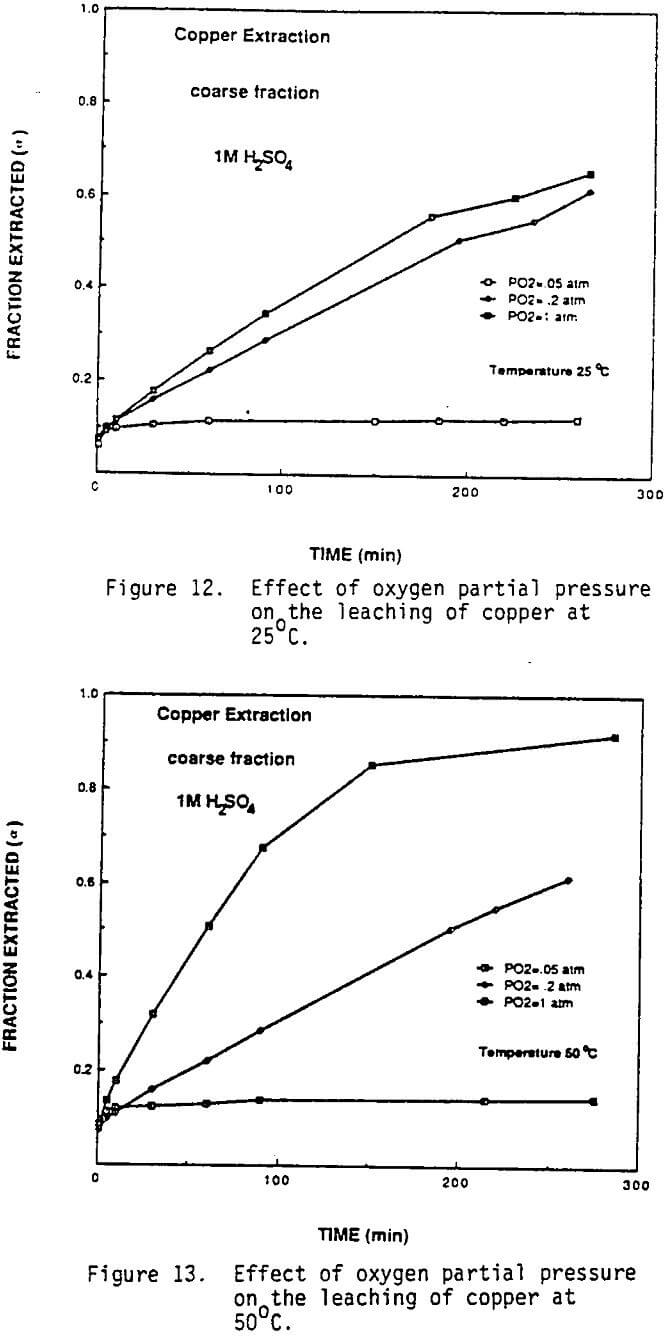
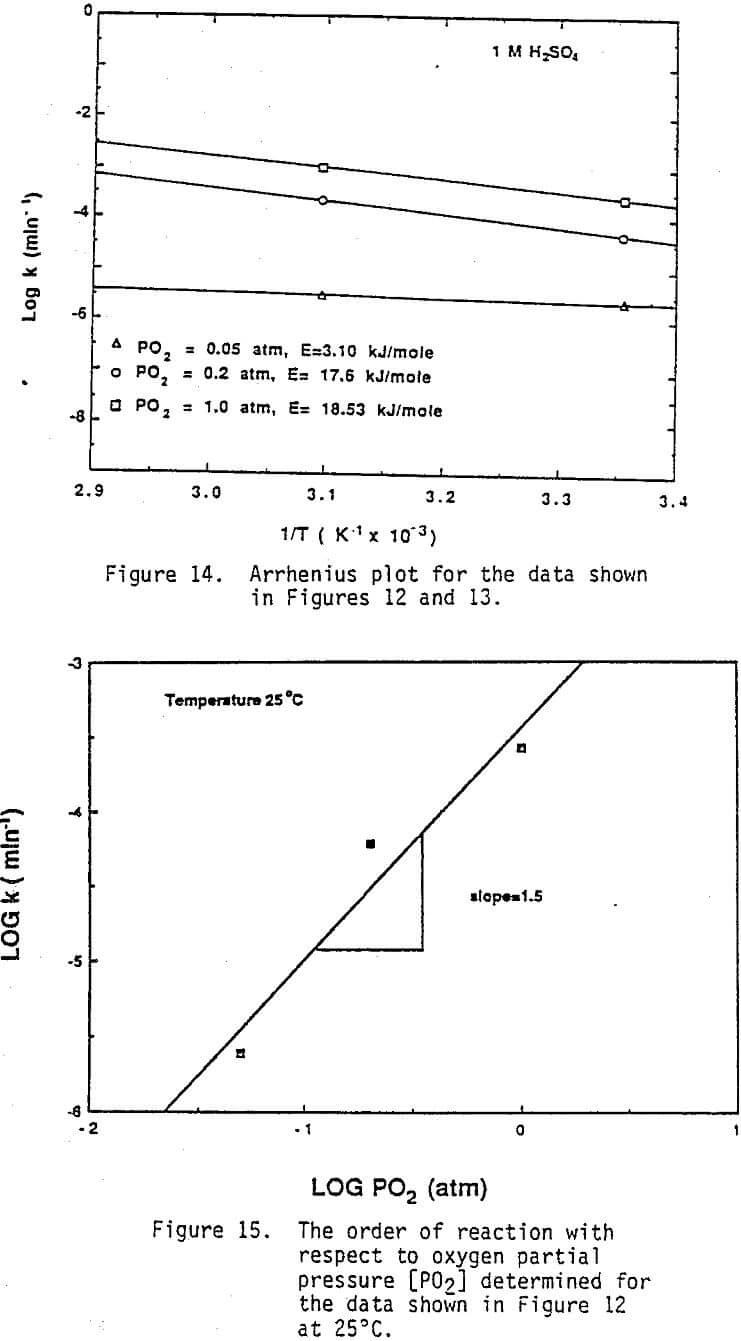
Effect of Oxygen: As-received material and the residues from several leaching experiments were examined under SEM. Typical photomicrographs are given in Figures 10 and 11. SEM and energy dispersive x-rays analyses revealed the presence of brass-like material containing significant amounts of both copper and zinc in the as received material and the leach residue, although the amount of metallic materials appeared to be less in the residue. Other particles in the as-received flue dust were found to contain primarily zinc and were assumed to be zinc oxide. In addition abraided and fractured grains of silica typical of used foundry sand also were identified. Most of the copper oxides and especially zinc oxide would dis¬solve fairly rapidly during acid leaching. Metals and alloys generally would be expected to leach much more slowly in the absence of an oxidizing agent. This was confirmed by the SEM and x-ray results in that numerous particles were identified as metal or metal alloy in the residue out no particles could be identified as primarily zinc (i.e., zinc oxide). As a result a series of experiments were conducted where the leaching solutions were purged with nitrogen, air or oxygen in order to vary the partial pressure of oxygen and provide an oxidizing agent to accelerate the dissolution of the metallic fraction. As can be seen from Figures 12 and 13, the fraction of copper reacted was strongly dependent on the oxygen partial pressure. Almost 99% of the copper present in the coarse fraction was extracted by oxygen purging at 50 C, whereas only 45% of the copper is extracted under the same leaching conditions when open to air (Figure 2).
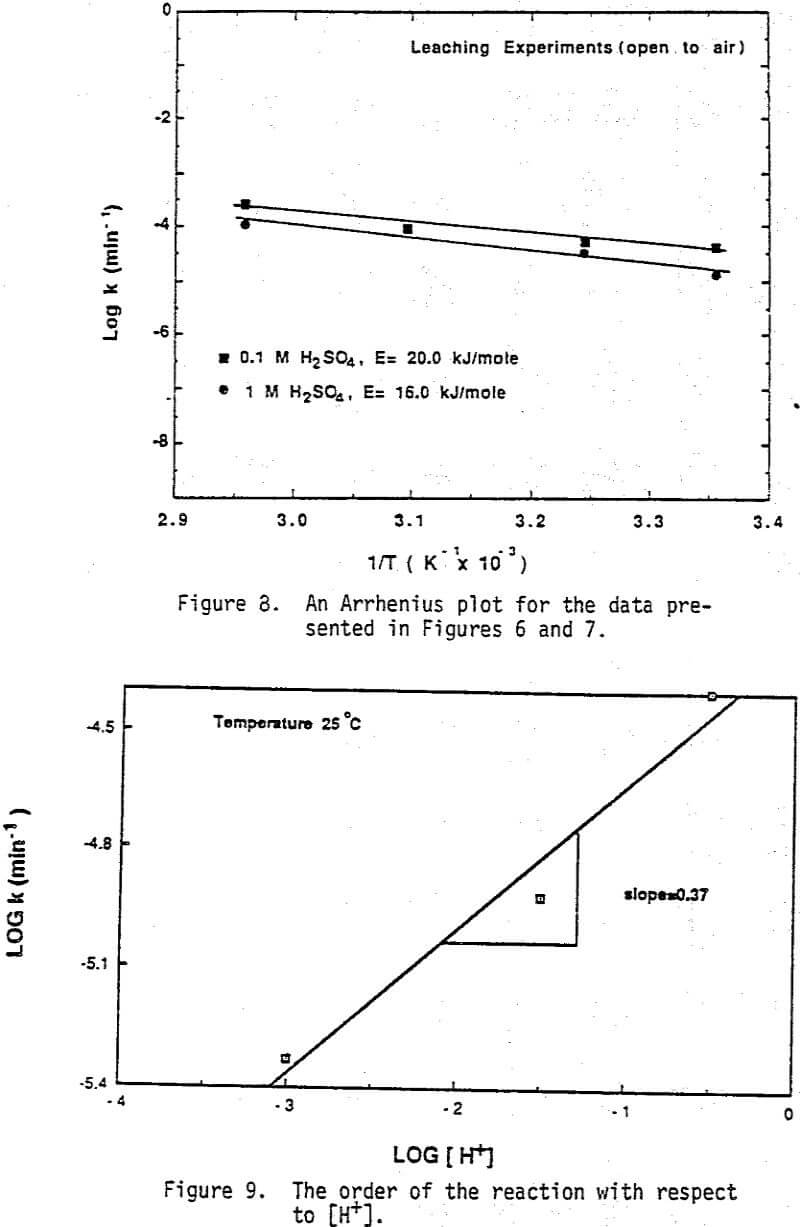
Arrhenius plots of the data shown in Figures 12 and 13 are presented in Figure 14 for each of the purge gases used. Although the accuracy of obtaining an activation energy from only two data points is not desirable, it is clear that all three experimental conditions yielded activation energies which were quite small. The activation energies were in the range of 07.-4.4 kcal/mole (3.10-18.53 kJ/mole) which were again consistent with a diffusion controlled reaction. By referring to Figures 8 and 9 it is obvious that the reaction order with respect to [H+] is 0.37, whereas the reaction order with respect to oxygen is approximately 1.5 (Figure 15). This indicates that the leaching reaction is more strongly dependent on oxygen concentration than on hydrogen ion concentration.