Considerable amounts of the world’s known reserves of elemental sulfur are found near the surface in areas of volcanic activity both active and inactive. Because such deposits cannot in general be mined by the well-known Frasch process, and because other methods of recovery have not proven to be economically competitive except in isolated instances, at present these reserves remain relatively undeveloped. However, projections of world sulfur demand indicate that new sources of marketable sulfur will be needed in future years.
Practical Aspects of Solvent Extraction
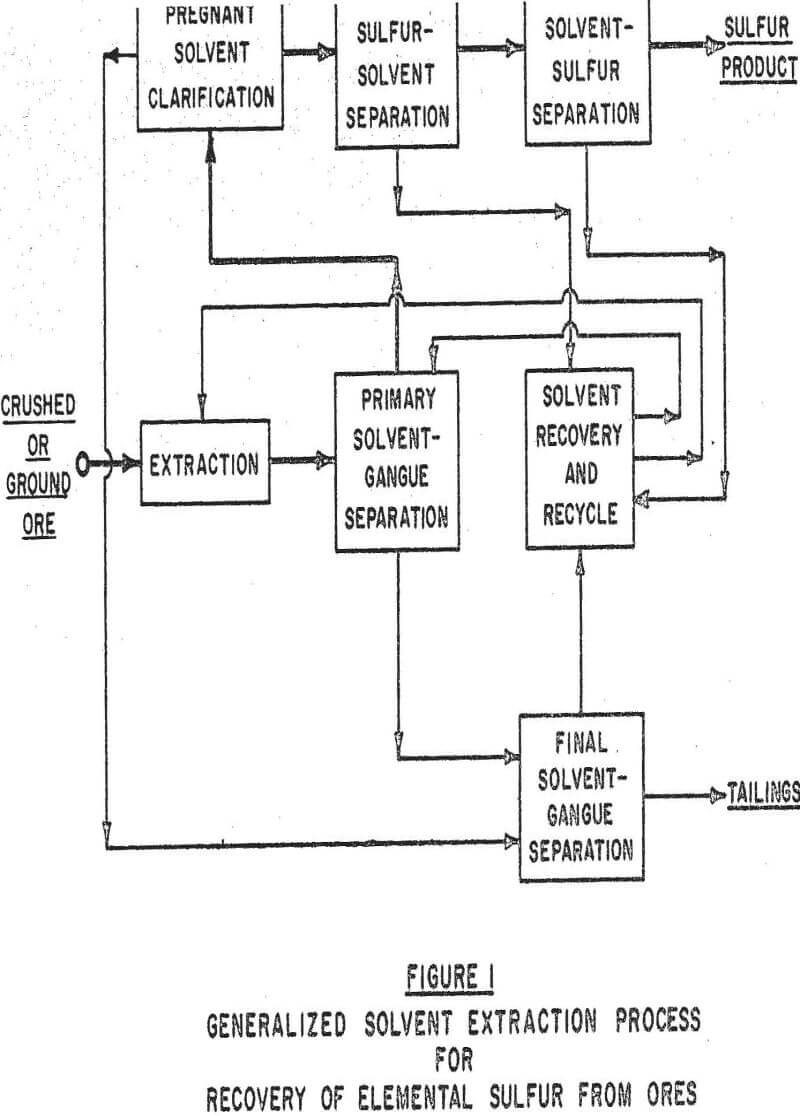
In general, in a solvent extraction operation elemental sulfur is dissolved from the ore by the solvent at an elevated temperature, the sulfur-solvent system is clarified of gangue, and solvent and sulfur are separated by crystallization of sulfur and/or vaporization of solvent to result in a sulfur product and solvent for recycle.
Native sulfur ores may fie quite acid by nature and usually contain water, thus begetting a potentially corrosive system. The presence of water can lead to additional effects both good and bad. Excessive water in the ore can block solvent penetration and thereby slow down the rate of sulfur dissolution in solvents which are immiscible with water.
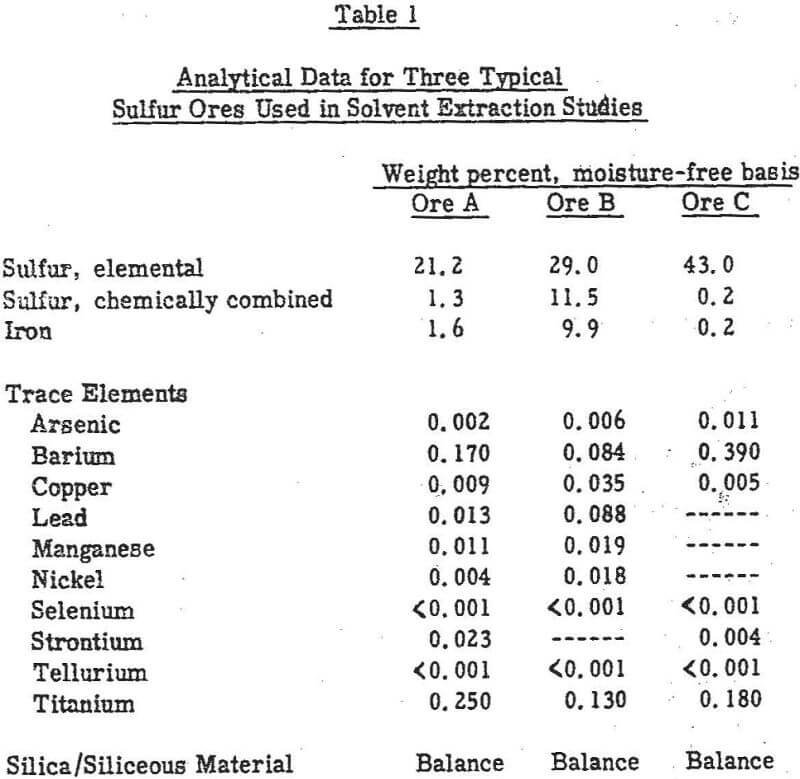
Of the many possible solvents, petroleum derivatives such as kerosene, aromatic hydrocarbons such as toluene and xylene, and chlorinated hydrocarbons such as trichloroethylene and perchloroethylene appear to be of most current practical interest. Carbon disulfide has received much attention in past years, and on the basis of information found in the literature is the only solvent to have been used commercially.
It is possible to obtain a sulfur product from a solvent extraction process either as crystals or in molten form. The feasibility of a molten product will depend on the degree of solvent solubility in the sulfur and on the ability of the solvent to withstand molten sulfur temperatures (above 235°F) without decomposition or reaction with sulfur. Formation of a crystalline product normally occurs when a loaded solvent is at a temperature below the melting point of sulfur. Super-saturation of the solvent is induced either by cooling or by adiabatic evaporation and cooling.
Pilot Plant Study of a Solvent Extraction Process
A solvent extraction process based on trichloroethylene may be among those used for recovery of sulfur from near-surface deposits on a commercial scale. The pilot plant has been designed to accommodate up to 1, 000 lb per hour of feed material. The plant consists essentially of a hot leg for leaching the elemental sulfur from the feed material to produce a sulfur-pregnant solution of trichloroethylene, a cold leg in which the sulfur is recovered by crystallization to yield sulfur-barren trichloroethylene which is recycled to the hot leg of the operation, and attendant equipment for recovery and recycle of barren trichloroethylene from leached residue and sulfur crystals. During the first twelve months of operation of this experimental plant, approximately 400 tons of feed material were processed to yield about 90 tons of elemental sulfur. Recoveries of sulfur from the feed material have averaged above 90%. The grade of sulfur produced has consistently averaged above 99.9%.