Table of Contents
The analytical procedure for determining the potential for a lead containing waste to be classified as a hazardous solid material was the E.P. toxic character test, which used an acetic acid extraction procedure. If the leach liquor from that test contained more than 5 mg Pb/l then the waste was classified as a hazardous waste. A new procedure, the toxicity characteristic leaching procedure (TCLP), which uses two different leaching solutions (acetic acid/sodium hydroxide; acidic acetic acid), was introduced in 1990, and is more rigorous than the E.P.Toxicity test. The cost of a TCLP extraction and analysis can typically cost over $200 per sample.
The cost of pyro or hydrometallurgical treatment of blast furnace slags is usually high due to the nature of the processes and the required capacities. Thus it is important that revenue be generated by the recovery of metal values during the treatment process. For lead blast furnace slags, it is therefore appropriate to recover the lead and zinc in separate solutions to subsequently permit effective recovery of both metals. If the metal values were not important then a bulk leach of the two metals or a simple lead leach would be possible to render the slags non-hazardous.
Experimental
Characterization of Lead Blast Furnace Slag
Initial characterization of the lead blast furnace slag received from Herculaneum was through a) wet chemical analysis b) scanning electron microscopy and c) particle size analysis. The chemical analysis was performed by dissolution of the slag in aqua regia followed by elemental assay by atomic absorption for lead, zinc and iron.
Lead and Zinc Leaching
a) Zinc Dissolution with Sulfuric Acid: To investigate the dissolution of zinc without lead recovery, a series of one hour room temperature leach tests were undertaken using acid concentrations ranging from 1M to 9M.
Finally a two stage leach test was carried out using 500 g of ground slag and 500 cc of 2M sulfuric acid for one hour at 60 °C. The first stage residue was then subjected to a second leach with fresh sulfuric acid leach solution.
b) Lead Dissolution with Various Leachants: Five different leachants were studied to determine the recovery of lead from the residue of the larger scale sulfuric acid leach which had removed most of the zinc and iron. The leachants were:
Sodium chloride solution (300 g/L)
Acidified sodium chloride solution (300 g/l NaCl + 3% HCl)
Sodium hydroxide (300g/l)
Ammonium acetate (300 g/l)
c) Lead and Zinc Simultaneous Dissolution : To identify a possible bulk leach system, hydrofluosilicic acid was used to leach both lead and zinc from the slag.
Solution Purification
Since the purification of zinc solutions by solvent extraction, ion exchange and precipitation has been covered extensively, this research concentrated on the solution purification and subsequent recovery of lead. The technique of solvent extraction was selected to purify the lead leach solutions produced by the acidified brine leach of the sulfuric acid leach residue.
Lead Recovery
A novel process of recovering lead directly from the loaded organic extractant using a solid reductant was evaluated. The test involved treating 10g of sulfuric acid leached residue with 100 cc of acidified brine.
Possible Process Flowsheet
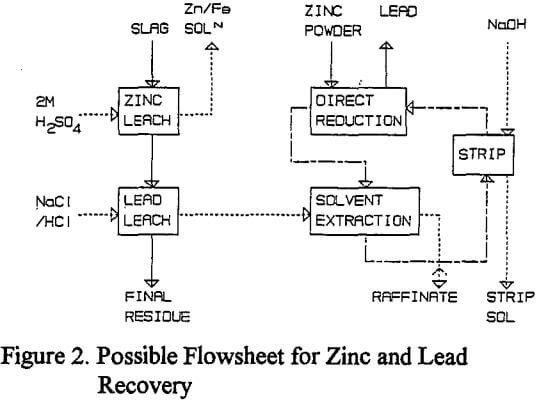
A series of tests were performed to demonstrate a possible flowsheet to recover zinc in solution, and directly reduced lead, from lead blast furnace slags. Initially a large scale sulfuric acid leach was performed to leach 900 g of ground slag for one hour at 60 °C using 9.01 of 2M sulfuric acid.
Results and Discussion
Characterization of Lead Blast Furnace Slags
The chemical analysis of the slags by AA gave 2.0% Pb, 13.0% Zn and 32.0% Fe, which fits the typical analyses generally quoted for these materials. Mineralogically the slags also contained 10.8% CaO, 4.7% MgO and 21.8% SiO2.
Lead and Zinc Leaching
a) Sulfuric Acid: The lead recovery is low due to the insolubility of lead sulfate and is not shown.
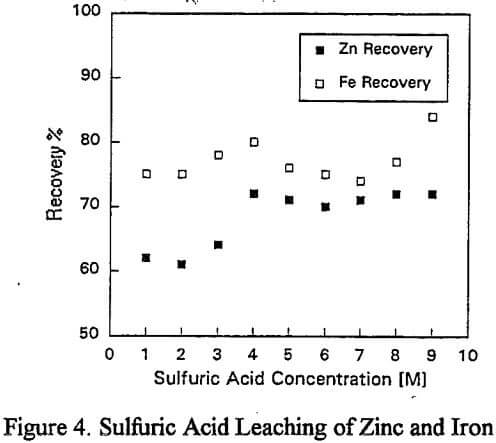
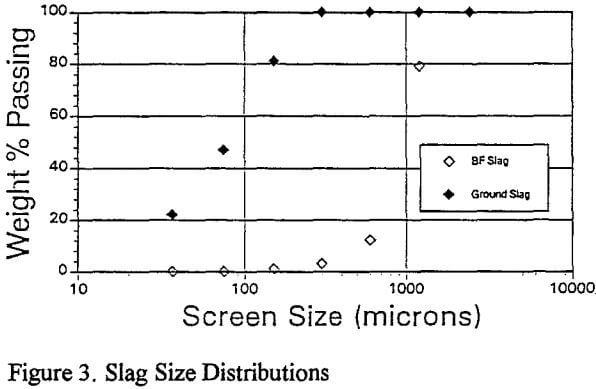
Replicate leaching tests revealed considerable metal dissolution variability (±5%) which was traced to the feed sample. As a result of rod mill grinding, the particle size was reduced to -0.3mm and metal dissolutions were reproducible to ±1%.
b) Lead Dissolution:
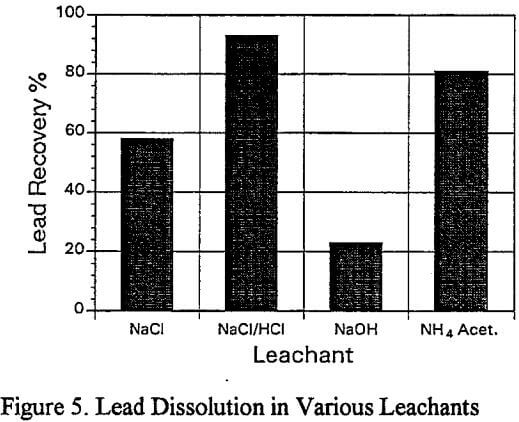
The acidified brine leach residue contained 0.4% Pb, 3.0% Zn and 0.6% Fe, and it is a apparent that these remaining metal values are strongly bound and would not represent a long term leach problem with respect to environmental concerns.
c) Hydrofluosilicic Acid Dissolution: The previous use of H2SiF6 to recover lead from battery sludges prompted tests with the lead blast furnace slags. The dissolution results indicate that this acid is equally effective with lead, zinc and iron, extracting approximately 80% of each under the test conditions.
Lead Solution Purification
The treatment of the lead containing solutions, after the sulfiiric/acidified brine leach, utilized solvent extraction to effect solution purification.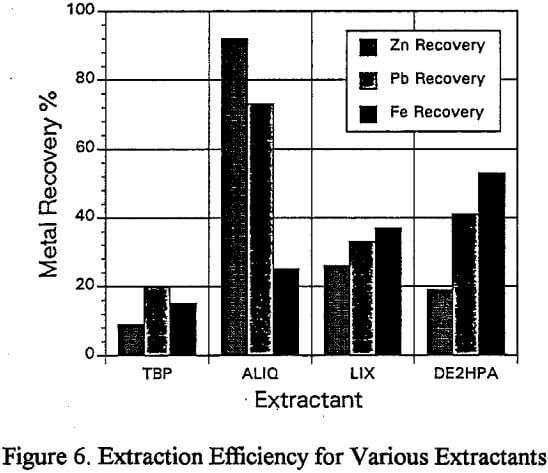
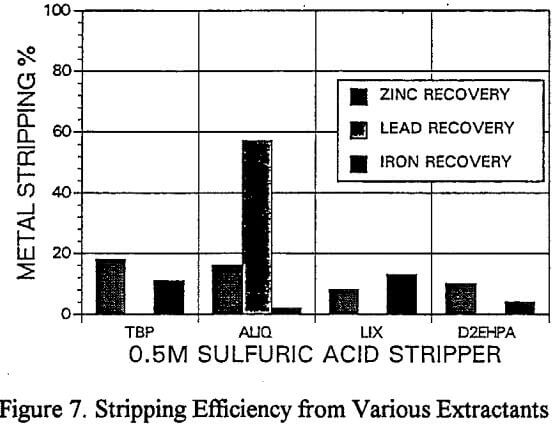
Lead Recovery
As a result of the initial problems with stripping lead from Aliquat, a novel process of directly reducing the lead from the organic phase by the addition of zinc powder was investigated. The direct reduction is thought to occur galvanically as illustrated by eqt. 1.
RPb +Zn²+ ↔ R Zn + Pb²+………………………………………………………………………………..(1)
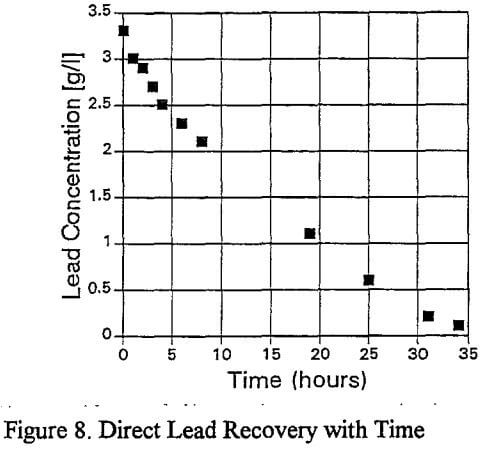
The lead is virtually completely removed from the organic after 34 hours. The direct reduction is considered to be electrochemical in nature, and the possible anodic and cathodic reactions are:
Zn (solid) ↔ Zn²+(org) + 2e………………………………………………………………………….(2)
Pb²+ (org) + 2e ↔ Pb (solid)…………………………………………………………………………(3)
Possible Process Flowsheet
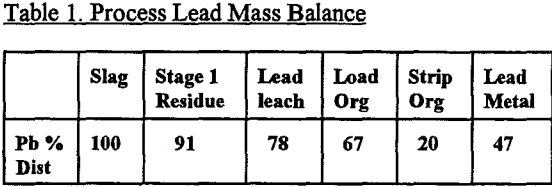
Using the treatment described in the experimental section, an overall selective separation was undertaken for lead, and the results are given in Table 1. It can be seen that the overall recovery of lead metal was only 47%, but 11% is contained in the brine leach solution and 20% in the organic solvent, both of which are recycleable. Some 13% of the lead actually remained in the solid residue, with 9% reporting to the zinc leach liquor.