Table of Contents
Copper mineralogy contributes a significant load of metals to the carbon adsorption circuit. Copper as [Cu(CN)2-] impedes adsorption of [Au(CN)2-]. If in high relative concentration, [Cu(CN)2-] loads faster than [Au(CN)2 ]; occluding adsorption space otherwise occupied by [Au(CN)2], Copper as [Cu(CN)3²-] and/or [Cu(CN)4³-] is weakly adsorbed onto activated carbon.
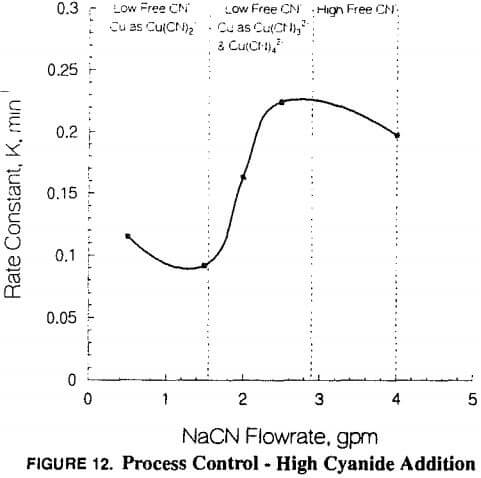
Conversion of copper to the higher coordination number cyanide complexes reduces adsorption interference; [Au(CN)2-] adsorbs at higher rates.
A brief summary of efforts undertaken towards copper interference control is as follows;
- Faster than normal carbon advance rate to accelerate gold removal
- Most efficient use of acid washing and regeneration for most active carbon adsorption media
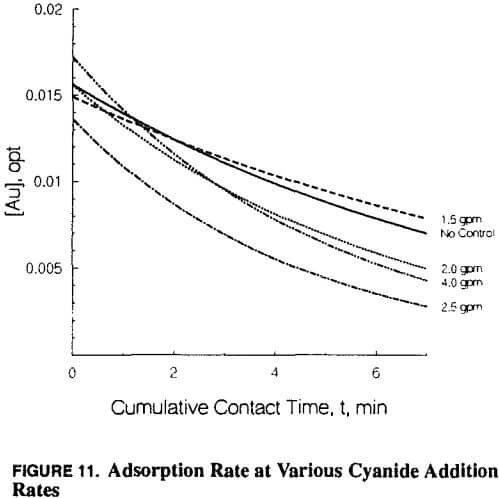
- Chemical control of interfering species such as higher than customary sodium cyanide concentration
- Process control scheme to allow operator to observe critical circuit parameters
- Correctly tie mineralogic information to all process areas
The specific use of pressure washing of electrowin sludge necessitated the limitation of copper sent to the electrowin circuit and subsequent deposition. Reducing copper sent to the electrowin circuit, and tighter control on electrowin voltage, makes washing of cathodes an efficient process with resultant modest copper content in the dore bars.
The study of the carbon adsorption circuit gives the operator significant room for improving efficiency of the plant.
Additional work is indicated to expand our knowledge base of other copper recovery methods from dilute copper sodium cyanide solutions at high pH.
Copper Recovery Methods
At the heap leach site, adjacent to the American Girl site where leaching commenced, copper problems were a significant factor in low carbon adsorption efficiencies. At that location efforts were expended at recovery of the copper metal to prevent further interference when hot strip and cold strip waste solutions were discharged to the heap leach solution system. The Scada system was implemented on a test basis to treat high copper content sodium cyanide solutions in an electrolytic copper reduction cell. Insufficient copper removal and very significant HCN gas evolution precluded ongoing use of the system. The other available systems were not tested. High flow rates and handling of HCN gas generation can limit the applicability of efficient copper recovery systems, especially at the high pH required for safe cyanide use.
Carbon Adsorption
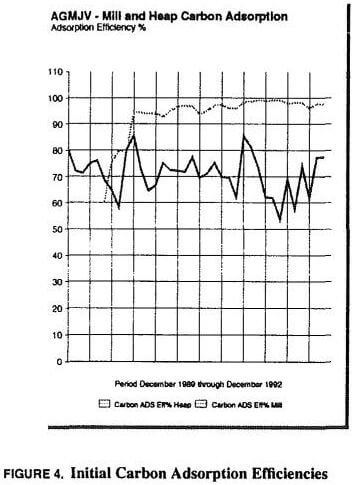
Carbon adsorption efficiency was unsatisfactory during the initial months of processing (see Figure 4.). Both mill and heap leach pregnant solutions were combined during initial start-up and pumped through a single bank of carbon adsorption columns. Bubble caps and solution distributor plates were scrutinized and additional bubble caps installed to enhance solution distribution. Little change was seen in column efficiency.
Regeneration
Thermal regeneration of the carbon was taking place in the early stages of site operation using the regeneration kiln with a capacity of 0.907 mt/day (1 ton/day capacity, vertical kiln). Activities were nominally monitored. Any carbon in excess of the capacity of the regeneration feed hopper bypassed the regeneration step.
This accounted for one-half of the total carbon moved daily. Significant problems occurred at times involving carbon hang-up in the kiln where overheating and ashing occurred, ultimately deteriorating carbon performance. In order to improve carbon adsorption performance an additional bank of carbon adsorption tanks was installed to handle the flow from the mill pregnant solution.
Additionally, a new strip vessel, second boiler and strip solution makeup tank, and second electrowin circuit were added. At this point, carbon advances numbered three per day (two each at 12 hours for mill carbon and once per day for heap carbon) and stripping batches and carbon movement significantly exceeded thermal regeneration capacity. Increased efforts at improving carbon regeneration throughput and control met without success. And, with more in-depth monitoring of regenerated carbon activity, the process was obviously being pushed too far beyond capacity. Replacement of the carbon throughout the circuit with fresh carbon renewed the adsorption efficiency briefly, and documentation of apparent density and activity showed that poor adsorption performance could not be attributed wholly to carbon activity.
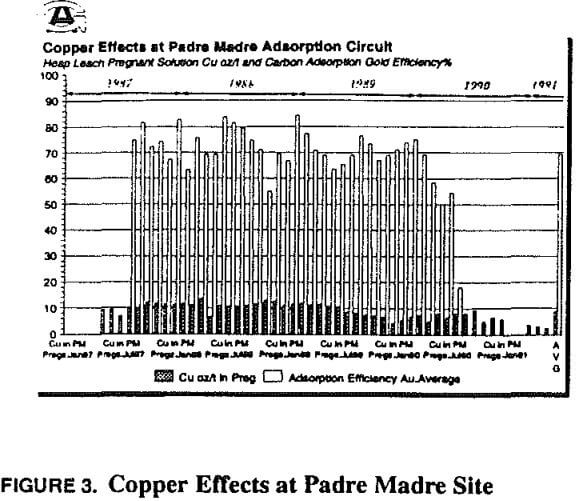
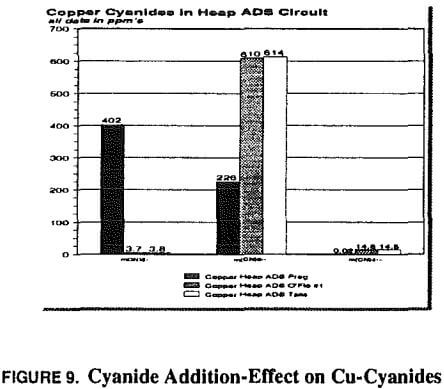
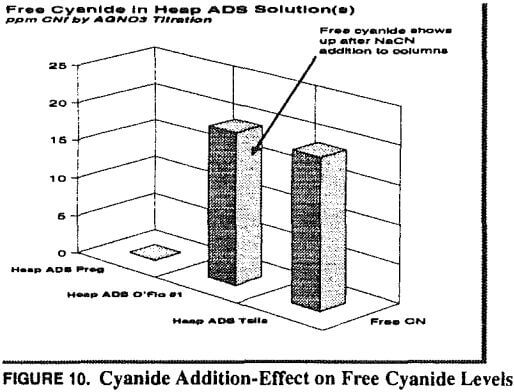
At this point copper became the primary focus, and all problem-solving efforts focused on copper. Copper-to-gold ratios were checked and demonstrated to be at a critical level (in excess of 600 Cu: 1 Au) in the head column and increasing to very high levels (several thousand to one) in the tail column. Cyanide levels in the heap leach barren solution were scrutinized to determine if anything could be done to reduce the leaching rate of copper and maintain favorable conditions for gold leaching. Cyanide-to-copper concentrations would be required in excess of 3:1 respectively. Current levels are already approximately 0.38 to 0.45 kg NaCN/mt (0.75 to 0.90 lb NaCN/ton) solution, in excess of those at heap operations where copper is not a significant problem. When copper levels in solution rise to 800 ppm, the cyanide requirements would be nearly 2400 ppm or over 2 kg/mt (4 lbs per ton). The thickness of the heap pile contributes to lowering of the cyanide through dissolution of cyanide soluble minerals, as previously described. With these cyanide requirements in mind, little hope was held for reducing cyanide to limit copper dissolution while maintaining proper cyanide for gold leaching.
Cold Stripping
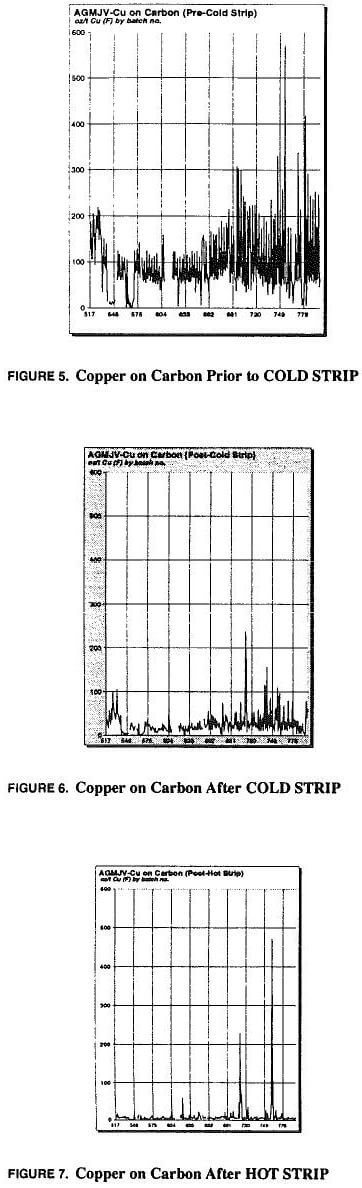
Use of ambient temperature high concentration sodium cyanide solution to drive off copper loaded onto activated carbon was instigated early in the process investigations. As stated in the circuit description (above) there were sufficient information sources as to the validity of a high cyanide pre-soak to remove copper prior to hot pressure strip (Muir, LaBrooy, Cao, 1989, Randol, 1987). A complicated arrangement was implemented, in which each tonne of carbon (from either mill or heap circuit) was cold stripped 3 times for 5 to 5½ hours per cycle. This was an excessive cyanide consumer, and was eventually optimized to a single batch cold strip for 1½ to 2 hours per mill or heap tonne. Figures 5, 6, 7 show the response of copper on activated carbon from the circuit to the cold stripping and hot stripping processes. Also shown is copper in the cyanide rinse effluent from cold strip.
Carbon Advance Rate
The carbon advance rate for the heap circuit was increased to remove gold more quickly from the circuit. This rate increase assisted in the abatement of bumping gold from the carbon sites (Sheya, Palmer 1989) by removing carbon from the circuit before extensive copper bumping occurred. The cost of processing the carbon frequently in both strip circuits (cold and hot) at low gold loadings was determined to be less than the high solution gold losses when carbon advances occurred only every 24 hours, as practiced before this change. This bumping effect is similar to a common occurrence with silver in heaps approaching closure during attempts to remove the last gold from the heap pregnant solution. Frequently, gold solution tails cannot be reduced due to silver affecting the adsorption onto carbon in an identical manner. The faster advance rate facilitates reduction of the solution tail by approximately 15%.
 |
 |
 |
 |

