Table of Contents
The possibility of cheap electric power for a number of mining areas in this country has suggested to many the idea of electric smelting of nonferrous ores in small units at or near the mines.
The electric smelting of nonferrous ores was tested fairly thoroughly 20 years ago by United States Bureau of Mines and by others, and the fact that it did not achieve continuing workable, success in any instance is sufficient proof that it offered no advantages at that time.
Lyon and Keeney thoroughly investigated the problems, covering the possibilities with iron ores, copper ores, lead ores, zinc ores, complex. sulphide ores, gold and silver ores, rare-metal concentrates, the making of ferroalloys, the construction of furnaces, the cost of power, and conditions under which the electric furnace might be used. They decided that, excepting iron ores, aluminum, and ferroalloys, electric smelting was in the experimental stage.
It is the purpose of the present paper to survey the technical and economic changes that have taken place and to determine whether electric smelting of nonferrous ores offers any more promise today than 20 years ago. The following factors need to be considered especially:
- Relative cost of heat from electric power and heat from coke.
- Ratio of value of recovered metals, especially of gold, to smelting cost.
- Improvement in milling practices, especially differential flotation, and its bearing on use of electric furnaces.
- Advances in technology that make electric heating more attractive.

While most of the current interest in electric smelting relates to pyritic gold ores, actually more data are available on other ores, notably zinc and copper. Accordingly, we shall consider the status of electric smelting of several types of ores individually, based on the factors enumerated.
Zinc Ore Smelting
In 1916, Lyon, Keeney, and Cullen reviewed the status of electrio-furnace zinc metallurgy in the following terms:
In the metallurgy of nonferrous metals, the electric smelting furnace has had a greater application for the treatment of zinc ores than in the metallurgy of any of the other nonferrous metals except aluminum the process has not been applied to any great extent because of the difficulty of condensing the zinc vapor produced in smelting in the electric furnace. The cause of this difficulty has not yet been definitely determined. With few exceptions the work has not been done on a very large scale, and so it may be said that the electric smelting of zinc ores is still in the experimental stage.
The foregoing note on smelting zinc ores is followed by a description of the electric-furnace process in Sweden. Roasted zinc-lead-silver slime was being smelted, but many difficulties presented themselves, although it was expected that they would be overcome. Current cost $10 per kilowatt-year, or 0.11 cent per kilowatt-hour.
In 1923 the Bureau of Mines again interested itself in electrothermic zinc smelting.
O’Harra’s report contains 11 pages of references on the electrothermic metallurgy of zinc. Electrothermic or thermoelectric smelting may be defined as the reduction of an ore by means of an electric current. It may be said that more than two-thirds of the zinc metal produced in the world is derived from the retorting or distilling of roasted concentrates; the rest results from the electrolytic or wet treatment of ores. For retorting, the heat, which is applied outside, is derived from natural gas, producer gas, waste heat, coal, or other combustibles. Retorting is not smelting; it is not simple, and losses are rather high. Neither is the smelting of zinc ores simple; in fact, in general it is impracticable.
The first electric furnace for the reduction of zinc ores was devised in 1885. Little was done then until 1900, through to 1915; but the production of zinc as metal was difficult, most of it being condensed as blue ponder. Later, the electrolytic treatment of ores more or less directed attention away from electric smelting. Because of the imperfections in retorting and in electrolytic processes, the United States Bureau of Mines and the Missouri School of Mines and Metallurgy undertook an investigation into the electrothermic metallurgy of zinc. The following types of electric furnaces were found to have been tried by other workers and are described and illustrated in O’Harra’s report: Direct-resistance, indirect-resistance, radiating-arc, and buried-arc as continuous and intermittent types or as slagging and dry-distillation types.
The greatest obstacle encountered in all the attempts to smelt zinc ores electrically, and one that has caused the failure of many otherwise promising attempts, has been the difficulty of condensing the zinc vapor obtained from the smelting furnace to liquid zinc. In the earlier work, the major part of the zinc was invariably obtained as blue powder, which clogged the condensers and had to be remelted to obtain a marketable product.
Complex as is this problem, many complexities arise in smelting ores. Zinc is oxidized by the carbon dioxide formed; the zinc vapor is diluted by carbon monoxide and is sulphidized by sulphur and sulphur dioxide; also, fine dust is likely to be carried into the condenser by the rush of evolved gases.
O’Harra says this regarding the possibilities of electric zinc smelting:
The electric smelting of zinc ores for the production of spelter can now be termed metallurgically feasible. The question of cost then becomes of paramount importance. Up to the present time, the plants in Norway and Sweden are the only ones that have been able to achieve success. Little data on their costs are available, but the plants have been able continuously to maintain and expand their operations during more than 15 years. They are favored by having very cheap power available, and it is probable that their process would not be practicable in this country the United States. Judging from published reports, their metallurgical results are not as good as have been obtained in much of the work in this country.
The largest single item of cost in the electrothermic process is electric power. An electric smelter must be near cheap power. A conservative estimate of the requirements of a properly constructed, continuously operating furnace of 5 to 10 tons daily capacity would seem to be 3.000 to 3.500 kw-hr. per ton of zinc metal produced, smelting a cold charge, or 2,500 to 3,000 kw.-hr. with a preheated charge. This consumption should be lowered by larger and standard operations. Somewhat more power than that stated may be required by low-grade ores. In the Western States, 24—hour continuous power can be obtained at rates of 0.4 to 0.7 cent per kilowatt-hour and off-peak power as low as 0.25 cent.
It is metallurgically possible to recover at least 90 to 95 percent of the zinc from an ore in an electric furnace in regular operation. Also, most of the proposed electrothermic processes recover lead, copper, and the precious metals as bullion and matte or as a product in desirable shape for blast-furnace treatment. Low-grade and complex ores offer no difficulty in the electric furnace, and the presence of impurities is not detrimental.
The cost of an electrothermic zinc plant will be less than that of either a retort or an electrolytic plant if power is purchased. The process has the additional advantage that units of 10 tons are practicable.
O’Harra concludes as follows:
Each of the three processes -retort, electrolytic, and electrothermic – has its particular field and there are undoubtedly places in this country where the electrothermic process could be profitably applied.
In the 13 years that have elapsed however, it has been impossible to find any cases, either here or abroad, in which electrothermic zinc smelting has been profitable, in spite of some rather extensive trials, especially that at Trollhattan, Sweden.
The attempts to smelt zinc ores in the electric furnace in Sweden have been described by Landis. Gustav deLaval began to experiment in the field of electric zinc smelting in 1893 and by 1898 he had developed a practical but intermittent furnace at Trollhattan, Sweden. By 1902 he could smelt roasted sphalerite using coke as a reducing agent and lime as a flux and he applied for the first of a long series of patents. In 1903 a company was organized, and large plants were built at Trollhattan, Sweden, and at Sarpsborg, Norway. The former was much the larger, employing 22 furnaces,, each of 500 horsepower, during the World War. The early furnaces were single-phase, with bottom contact and one movable top electrode. The newer furnaces, which had greater hearth area, were provided with two top electrodes and no bottom connection. One of these was merely suspended in the furnace and lowered as consumed. Zinc was recovered partly as liquid metal and partly as powder. As developed to 1925 the Trollhattan process was one of all—electrothermics involving the use of four different types of electric furnace on zinc and a fifth for lead refining, if lead was present.
A few details of the process follow, as given by Landis:
The sulphide ores ware roasted in a multihearth furnace and mixed to make the charge as nearly self—fluxing as possible.
The smelting operation was carried out in a closed, arc-resistance type of electric furnace. The reaction was essentially one of reduction and volatilization, the gangue of the ore being fluxed to produce a fusible slag. The furnaces were charged from overhead bins, the charge consisting of a pre-mixed roasted ore, flux, and coke breeze; and if copper was present in the ore, enough sulphur was left in the charge to produce a-low-grade matte. This smelting furnace handled all off-grade slags and mattes, together with any residue of zinc dust not liquefied in the rotary furnace and the various residues from the refining furnaces.
The furnace operated in the smelting zone at a temperature above 1,400° C. (2,523° F.), and lead and zinc were volatilized and left the furnace in vapor form mixed with carbon monoxide. This gas stream passed to water-cooled condensers, where the metals dropped out in the form of a powder and mechanical screw conveyors were provided for continuously discharging the powder through a suitable seal.
The carbon monoxide passed out through a water seal and burned at the exit stack. The character of this flame was an important guide to the furnace operator.
The slag and matte together were tapped from the furnace into slag pots, where the matte settled to the bottom and was separated by the older system of “muscular metallurgy.” Gold and silver present in the ores appeared largely in the matte, although some silver was volatilized and recaptured at a later stage in the process.
The zinc-lead powder removed from the condensers was collected in metal containers, which were carried to bins placed above the rotary furnaces. In these electrically heated furnaces the powder was given a rolling and tumbling at a temperature well above the melting point of zinc (759° F.). A large part of the powder was rolled into liquid, which was tapped from the rotary furnaces at intervals. The unliquefied powder was mechanically discharged through the axis of the furnace and, after cooling, was carried back to the ore- smelting furnace; hence the name “return powder.”
Eventually, the lead-zinc alloy was liquated and yielded refined zinc slabs and lead pigs, also an iron-silver-copper cake that was sold.
The power consumption, calculated back to the original zinc ore from Burma, was 860 kw.-hr. per ton. The total consumption per metric ton of 57 percent material on the three furnaces was 3,335 kw.-hr., or, per ton of zinc recovered, 6,530 kw.-hr.
Landis makes this statement:
In spite of years of work recorded in volumes of literature, and an expenditure of large sums of money, there is not a single electrothermic zinc smelter operating anywhere in the world. In this industry the electric furnace plays only a secondary part today.
The Trollhattan enterprise was at a standstill in 1936.
The only electric zinc furnaces operating in the United States are those at Josephtown, Beaver County, along the Ohio River, Pennsylvania. They make zinc oxide and are operated by the St. Joseph Lead. Co., which treats 57 percent zinc concentrates from its Edwards mine, St. Lawrence Co., N.Y. In the plant are two Herreshoff 12-hearth roasters, three Dwight-Lloyd sinterars, Cottrell precipitators, sulphuric-acid equipment using vanadium pentoxide as catalyst, and preheaters, eight Gaskill patented electrothermic furnaces arranged to make baghouse zinc oxide, which is advertised as lead-free. The company annual report for 1933 said: “The electric furnaces have proved their eminent suitability for the production of lead-free zinc oxide.” Power is supplied by the Duquesne Light Co. plant near Pittsburgh at 66,000 volts. Six 1,500 kw.-amp. transformers reduce this to 2,300 volts, and each furnace is served by three 2,300/320/160-volt, 1,800—ampere, single-phase transformers. The process is described by George F. Weaton in volume 121, Metallurgy of Lead, and Zinc, American Institute of Mining and Metallurgical Engineers, 1936. The plant was designed to treat 120 tons of concentrates daily.
The furnaces have a bore of 57 inches and are 37 feet over-all in height. The sinter cake, assaying almost 60 percent zinc, is broken to 4— inch size and smaller, and with coke of similar size is heated to 750° C. by natural gas and fed to each furnace, which contains 25 tons or more. Electrical contact with the charge is made by means of three pairs of carbon electrodes; the charge itself constitutes a resistor 57 inches in diameter and 24 feet long. Between the rotating discharge table and top electrodes the temperature is approximately 1,200° C. About 18 hours is required for the passage of the charge through a furnace, each of which consumes 29,000 kw.-hr. daily. The voltage averages 265.
The final product varies according to customers wishes, but a typical example is 99.4 percent zinc oxide, 0.01 to 0.05 percent lead oxide, 0.002 percent each of cadmium oxide and ferric oxide, and 0.06 percent sulphur as the trioxide.
As is seen by the foregoing description, these electric furnaces are not actually smelting the zinc concentrates; but use is made of electric heat to volatilize the zinc which is condensed as oxide. The coke serves a double function – to provide the resistance oath for the electric current and the carbon for reduction of the zinc.
The refractories used in the electrothermic furnaces are described by Winfield B. MacBride in the Bulletin of the American Ceramic Society for December, 1935. It was found that electrical features of the furance introduced more than the usual limitations in refractories. The furnace wall is composed of circular sections of refractories supported on steel skew-rings, which are electrically insulated from the furnace columns. The principal slagging elements that affected the refractories were iron oxides and iron-zinc silicates of a fusing temperature of 1,120° C.
https://www.youtube.com/watch?v=Eo2ZGy1OrlI
The major refractory problems pertain to tho silica and iron solution attack at high temperature., expansion, porosity, gradual, disintegration due to zinc penetration, and cost. Exact dimensions of shapes is of importance, also. Experimentation finally evolved a mix of low-alumina grog (minus 10-
mesh, with control of the amount of minus 100-mesh), a ball clay, and 9 to 10 percent water. The shapes are fired to 1,430° C. The texture is uniform, the refractory has a metallic ring when struck, and the porosity averages 15 percent. Low porosity is an important feature in any refractory for this type of furnace.
The possibilities of metallic-zinc production by reduction with solid fuel and electric heal would seem to have been fairly well exhausted. The economic possibility of the natural-gas reduction process developed in the Metallurgical Division of the Bureau of Mines remains to be determined, however.
Briefly, this process, which was first tried in 1930 is as follows :
Maier, of the Bureau of Mines, had discussed the use of methane for reducing zinc oxide and predicted equilibria at various temperatures for the more important reactions. Doernor extended the study.
The gas used contained 86.85 percent CH4, 7.86 percent C2H6, 3.87 percent and smaller amounts of the higher homologs. The reducing power by reactions analogous to the reaction ZnO + CH4→Zn(gas) + CO + 2H2 is proportional to the carbon content, which in this case is about 20 percent higher than that of methane; also, about half of the reducing power of the higher members was directly utilized.
The experimental retort need not be described. It was charged with sintered concentrates assaying 69 to 70 percent zinc, calcines with 68 percent zinc, and zinc dross. All gave satisfactory results. The condensation of the zinc presented difficulties not encountered in current retort practice, and a greater condensing surface was needed because of the lower concentration of zinc vapor.
A recovery of 95 percent is feasible, most of the zinc being recovered as a metal, and 5 cubic feet of gas will produce about 1 pound of metal.
The technical possibility of the process has been demonstrated in the laboratory but its economics must be proved on a working scale.
Smelting Zinc Ores
To review the status of electric zinc smelting in regard to the four factors enumerated:
- Because some solid or gaseous reducing fuel is necessary, and that for heat need not be expensive coke, electric heat must be cheap indeed to compete with combustion.
- No type of electrothermic smelting of zinc lends itself readily to precious-metal recovery, although natural-gas reduction would seem to be advantageous.
- Milling improvements make it generally unnecessary to consider complex ores, as at Trollhattan.
- The only advance in technology that would seem to affect the situation is the proposed natural-gas reduction process.
Copper Ore Smelting
In considering the smelting of copper ores, Lyon and Keeney said, in 1915:
The electric smelting of copper ores is nothing more than the substitution of electric heat for the heat derived from the combustion of carbon. Inasmuch as the carbon which is used either in the reverberatory furnace or in the blast furnace plays no important part in the reactions that take place in these furnaces, there is no reason, metallurgically, why electric heat may not be substituted for the heat derived from the combustion of carbon. In fact, in some cases the.reactions would take place to batter advantage in the neutral atmosphere of tho electric furnace than in the reducing or partly reducing atmosphere of the combustion furnace. Therefore, the practicability of using the electric furnace for the smelting of copper ores would largely depend on the relative cost of coke and electric power.
As the use of the electric furnace is not advocated as a competitor of the combustion furnace, but as a substitute for it, in those localities where it is not advisable, because of the high cost of fuel, to use the combustion furnace, but as a substitute for the combustion furnace where conditions are such as to warrant its use, especially in the treatment of copper—bearing ores.
In this connection it is to be remembered that the development in the electric furnace in the iron industry for the reduction of iron from its ores was due to necessity. As a matter of fact, the field for the electric furnace in the reduction of iron ores is limited. Perhaps the same is true as regards the possible application of the electric furnace to the treatment of copper ores; but, judging from the comparative costs, it would seem that the possibilities of the electric furnace for tho treatment of copper ores are greater than those for the treatment of iron ores, because there is not so great a difference between the cost of coke and of electric power in copper-mining districts as there is in iron-smelting centers. Also, the cost of electric power is constantly becoming less, through improvements in gas engines and steam turbines, so that in districts whore water power is not plentiful but cheap fuels unsuited to coke-making are available it may be found more advantageous to use electric heat than the heat derived from the combustion of coke.
The report just cited presents, first, a critical discussion of the possibility of smelting copper ores in the electric furnace; second, the results of the experimental work of other investigators on the electric smelting of copper; third, the results of experiments by the authors on the electric smelting of native copper concentrates and sulphide copper ores; and fourth, a comparison of the electric, furnace with the blast furnace and reverberatory furnace for copper smelting.
In general, there are three classes, of ores that have to be considered in copper smelting – native copper; the oxides, silicates, and carbonates; and the sulphides. The third-named presents additional complications.
A number of runs were made with an electric furnace smelting a fine Michigan native copper concentrate, and it was concluded that such material could be smelted to produce a good-grade of black copper without excessive loss of copper in the slag or otherwise.
To attain the greatest economy in such work, an electric furnace must be operated continuously. The smaller the scale of operation and the more frequent the interruptions, the greater will be the metal losses. A furnace proposed for reducing native copper concentrate would have a smelting chamber lined with refractory brick, three carbon electrodes suspended vertically through the roof, and a charging stack lined with firebrick over the crucible. In 24 hours 750-kw furnace of this type should smelt 23 tons of such concentrates assaying 25 to 40 percent copper.
Lyon and Keeney then discuss the smelting of sulphide copper ores in the ordinary blast furnace end the possibility of substituting the electric furnace. They tell of the reduction of copper ores in Chile and in Europe more than 30 and 20 years ago. Then follow the experiments of the Bureau of Mines in 1915 whose objectives were:
- To determine if there were any conditions under which electric smelting without air would be anything but a simple melting operation.
- To note the percentage of concentration and the sulphur removal.
- To study the possibility of condensation of the elemental sulphur as such.
- To get general figures on power consumption with varying charges
- To determine losses of gold, silver, and copper.
- To study the use of a low-grade copper matte as a collecting agent for gold and silver.
The furnace used by Lyon and Keeney was 11 inches square and 15 inches deep, lined with firebrick and with a carbon bottom. The crucible, which had a taphole, was supported on an arch of firebrick. A 2-inch graphite electrode was lowered into the top of the crucible and one of like area protruded from the bottom. The top was roofed and kept closed to prevent escape of sulphur and admission of air.
Low-grade sulphide copper ore high in sulphur, a gold- and silver-bearing siliceous ore, and some roasted ore were smelted. Twenty runs were made, and the average recovery of five of them was 95 percent of the copper, 90 percent of the gold, and 75 percent of the silver. The metals were collected in a matte and the slags contained little metal. About half of the gold lost was lost mechanically, and a large part of the silver loss was mechanical and by volatilisation. Electrode end power consumption were high, but in a furnace of proper design and operation these would be much lower. Costs are discussed – these of the electric furnace with those of the blast furnace.
Lyon and Keeney covered much the same ground in their paper, The Smelting of Copper Ores in the Electric Furnace, which is to be found in Transactions 47 of the American Institute of Mining Engineers, 1914. A fair amount of discussion followed the paper.
Experience in Norway
According to Witherell and Skougor, large-scale experiments or campaigns in Norway showed that electric smelting is practicable where the necessary power is available at low cost. The choice of smelting by electricity or by regular furnaces is a choice of lower power cost as against high fuel cost. The metallurgical advantages include sulphur conservation, reduced metal loss, and smaller flux; requirements than for reverberatory furnaces. The electric furnace is suitable for ores low in sulphur.
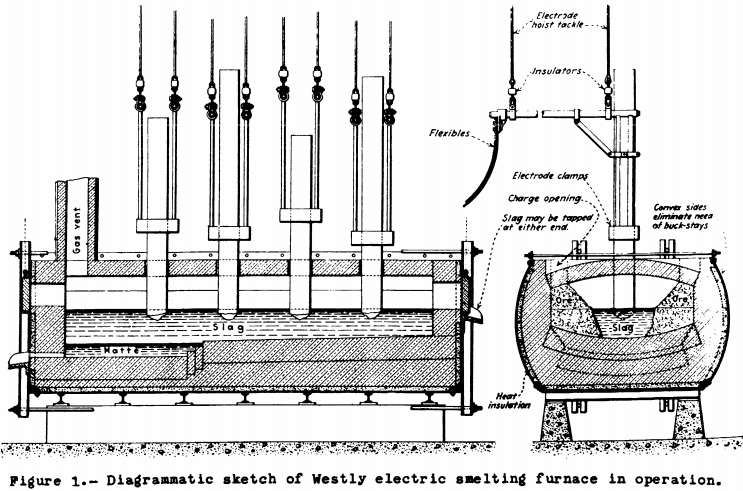
At Sulitjelma, Norway, the following quantities of copper material were electrically smelted in the Westly furnace designed by Jens Westly, metallurgical engineer for the Sulitjelma Copper Co.
1917 – 2,139 tons of raw concentrates and 2,747 tons of roasted concentrates; 1918 – 1,817 tons of raw concentrates and 31187 tons of roasted concentrates;. 1919 – 216 tons of raw concentrates and 546 tons of roasted concentrates. War conditions in Europe ended regular operation, including the construction of a 3,000—kw. furnace to smelt 100 tons per day and satisfactory trial runs on zinc ore.
The Westly furnace (Fig. 1) consisted of a nontilting boiler – iron shell lined with standard refractories and with an open arched roof with regular electrode openings. The electrodes were lowered vertically through the top. There were three to six of them in line, depending on furnace capacity. Their tips dipped into the slag layer but were kept well above the matte layer. This arrangement takes advantage of the fact that all mineral substances that are electric insulators when cold become high-resistance conductors when above red heat. There was no arc action in normal running. Electrode consumption was 4 kilograms per metric ton of material smelted.
The Westly furnace, used 3-Phase, 50-cycle alternating current at 112 to 230 volts. The several sizes were of 200- to 900 kw. capacity, and the power consumed in the large furnace was less than 700 kw.-hr. per metric ton of ore smelted. No other fuel was used.
Ore from the Sulitjelma mine consisted of copper pyrite, iron pyrite, and pyrrhotite. This was partly concentrated, giving a product carrying 6 percent copper, 28 to 30 percent sulphur, and 28 percent silica. Part of the concentrate was roasted and mixed with the raw portion before it was charged into the furnace. The matte assayed 30 to 40 percent copper and the slag 0.3 to 0.4 percent.
Electric Furnace Smelting Practice
The most recent plant to smelt copper ores electrically is that at Imatra, Finland, and we are indebted to Eero Male in en, general manager of Outokumpu O. Y., for the following information based on questions asked him through George Orr, United States Consul at Helsinki (Helsingfors). The company officials are satisfied with the furnace and will show it to anyone interested.
The electric furnace is of the resistance type, the slag overlying the matte being the resistance. It is cylindrical, measuring 10 meters (33 feet) in diameter and 4 meters (13 feet) in height. The bottom and walls are magnesite brick, and the arched roof is “chamotte.” The side, on which are four holes for tapping matte, is jacketed with water-cooled copper castings. Otherwise the furnace shell is steel. The three electrodes are of the continuous Soderberg type, each 1,400 millimeters (56 inches) in diameter. The electric current is 3-phase 50 “cycle” with 90-110 volts between the electrodes, and the power load 7,000 kw.
The percent copper ore from the mine is dressed by flotation. Concentrates assay 22 to 24 percent copper and have a fineness of 80 percent through 200-mesh. Tho daily output is 150 metric tons dry weight, and the concentrates are dried to 4 to 5 percent moisture in a rotary kiln.
One—half of the concentrates are roasted in the sulphuric—acid plant, and the calcine is mixed with raw concentrates, lime, and quartz sand without briquetting or agglomeration. The change is as follows:
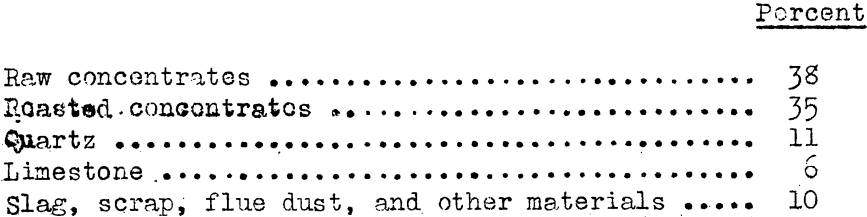
This mix is charged into the furnace, the daily capacity of which is 250 to 300 tons of dry materials, excluding converter slag. It operates continuously; charging is continuous, but tapping matte and slag is intermittent. The consumption of power is 540 kw.-hr. per tons of dry charge, the liquid converter slag not included.
The products of this electric furnace are matte, slag, and gas, just as from a reverberatory matte furnace. Matte carries 45 percent copper and is blown to 99 percent blister, as, customary. This is exported. Slag assays 0.3 percent copper, 57 to 58 percent iron oxide, 31 to 32 percent silica, 5 percent lime, and 1.5 to 2 percent sulphur. The sulphur dioxide in the smelting furnace and converter gases is absorbed, extracted, and compressed to liquid SO2, which is sold to sulphite paper-pulp mills.
Although the unit cost of power is not available, the cost of electric smelting of copper concentrates and converting to blister is 800 Finnish marks, or about $17.50 per ton of copper.
With regard to the price of power in large quantities, Edvard Svanoe, Oslo, Norway, stated in The Engineer ( London ) for dune 18, 1937, that electric power in that country may be obtained at 1/16 penny or- 1/8 cent per kilowatt-hour.
Smelting Lead Ores
Lyon and Keeney said, regarding lead ores:
The smelting of straight lead ores in the electric furnace has seemingly never been attempted either commercially or on a large experimental scale, largely because of the ease and cheapness of smelting such ores by combustion processes. For the smelting of ordinary lead ores, it [the electric furnace] has no especial application, but in the treatment of complex, sulphide ores the electric furnace might be profitably used.
No conditions would seem to have arisen that would, increase the applicability of the electric furnace to lead smelting.
Pyritic Gold & Silver Ores Containing Minor Elements
Lyon and Keeney stated, regarding gold and silver ores:
In the smelting of gold and silver ores carrying no lead or copper to form either a lead bullion or a copper matte, but containing iron sulphide, the electric furnace might be used in connection with an air blast for oxidation of the iron sulphide, an iron matte being used as a collecting agent for the gold and silver; a fair recovery can be made in this way.
This general principle seems to us to be sound. The matte, rich in precious metals, so formed could be shipped to the smelter; or as the miner much prefers to obtain bullion for shipment to the Mint, the matte could be cast in anodes and treated electrolytically for the recovery of the precious metals.
No methods have been, worked out for this type of smelting, and until they are we cannot recommend them to the small worker.
As its new experiment station at Boulder City, Nev., the Metallurgical. Division of this Bureau has installed equipment for the electric smelting of certain concentrates – copper-nickel for a start – but no results can be expected for at least several months. Runs will be made on ores of other metals. The findings will be published from time to time as results warrant.
Smelting Concentrates
With regard to the economics of electric power and fuel for smelting nonferrous ores and concentrates, mentioned early in this circular, the new smeltor of Marsman and Company, Province of Camarines Norte, Luzon, Philippine Islands, is an example: During 1936, flotation concentrates of two mines
which were shipped to the United States averaged as follows:
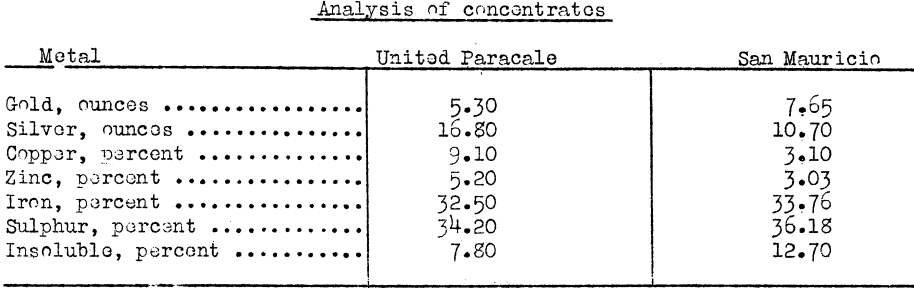
Selective flotation made it possible to produce a high-grade concentrate which could carry the cost of sacking, freight, and smelting charges.
It cost, including smelter deductions, $51.25 and $42.06 per ton respectively, to handle, ship, and smelt these concentrates. The charges on concentrates from the Suyoc mine were $70.42 per ton.
To reduce these costs, the local smelting of concentrates was considered. The reverberatory was eliminated because, of its excessive first cost and high operating cost, especially on the relatively small tonnage available – 30 tons a day at present, but custom business is expected. The electric furnace had to be rejected on account of the prevailing high cost of power in the Philippines. This left the blast furnace, and as raw concentrates are not desirable for such a furnace, it was decided first to sinter the concentrates, smelt the sinter, and ship the matte for refining.
The smelter commenced work in May, 1937. As received, concentrates assay 4.75 ounces of gold, 10.25 ounces silver, 4.25 percent zinc, 3.10 percent load, 41.50 percent sulphur, and 9.70 percent insoluble. They are mixed with a flux, distributed onto the 4 by 10-foot Mace sintering hearth, the sulphur ignited with a portable oil-burner, and the mass semifused. The sinter contains 40.9 percent FeO, 5.3 percent CaO, 8.2 percent S, and 32.6 percent insoluble.
Additional fluxes, when needed, are added to the charge, in the number 4 Mace blast furnace. Siliceous mine ores supply the silica and beach coral the lime.
Ten to 12 tons of concentrates can be reduced to 1 ton of matte which contains 40 percent copper and 40 to 60 ounces gold per ton. It is advantageous to ship matte to Tacoma.
It is estimated that a net saving of at least $15 per ton of concentrates will result from the local blast-furnace reduction of concentrates.
Electric Furnace Accessories
Following is a list of makers of electric furnaces, or equipment therefor, who would be glad to offer advice on furnace practice:
- Ajax Electric Furnace Corporation 1108 Frankford Ave.,
Philadelphia, Pa. (Furnaces.) - American Bridge Co., Ambridge, near Pittsburgh, Pa.
(Heroult furnace.) - Detroit Electric Furnace Corporation, 325 West Elizabeth
St., Detroit, Mich. (Furnaces.) - Electric Smelters, Inc., Central City, Colo. (Wile furnace.).
- General Electric Co., Schenectady, N. Y. (Apparatus.)
- Greene Electric Furnace Co., Seattle, Wash. (Furnaces.)
- Pittsburgh Lectromelt Furnace Corporation, Pittsburgh, Pa. (Furnaces.)
- Westinghouse. Electric Manufacturing Co., East Pittsburgh,
Pa. (Apparatus.)
Electric Furnace Metallurgy
While the electric-furnace metallurgy of iron, manganese, and chromium is not a main concern of this report, a short section on the present status of smelting those metals by electricity has been added:
Lyon and Keeney also gave considerable attention to the reduction of iron, ore and the melting of iron and steel in the electric furnace. The problems and practice to 1914 are thoroughly reviewed and discussed. No actual smelting of ore was done by the Bureau of Mines.
Apparently, the first electric furnace for smelting iron ore was tried in Italy in 1898. A few years later a Canadian commission visited Europe to study the reduction of iron ores in Italy, Branco, and Sweden, and in 1906 an electric furnace was tried at Sault Ste. Marie, Ontario. Results were reported as being satisfactory, but no large-scale smelting of ores has been done since.
Considerable space is given in Lyon and Keeney report to the electric smelting of iron ores in Sweden, which, we may say, is the only country in which such metallurgical work is being done on a large scale. The plant at Trollhattan cost $93,000 and the results were fairly satisfactory and improving. The ore carried up to 68 percent iron. Limestone was used, also some charcoal. Power consumption per ton of iron produced was around 2,000 kw.-hr.
The foregoing text is followed by a review of the problems in the electric smelting of iron ores and the status of the iron industry in the western United States. Prospectors rarely gave-iron ore a thought; they looked for iron only as an indication of the presence of gold and silver at the surface and copper at depth. Probably, in panning or otherwise testing iron outcrops, if no minerals showed the prospectors considered them valueless, not thinking of iron ore.
As is well-known, much experimentation on the electric smelting of iron ores was done in Shasta County, Calif., but nothing has been tried for 20 years. Now that power at 1-½ to 3 mills per kilowatt-hour (which is equivalent to $9 to $15 per-horsepower-year) will be available from the Columbia River, Oreg., the electric smelting of western iron ores is a revived project, but only on an extensive scale., Hodge devotes some space to this in a report made to the North Pacific Division, Corps of Engineers, United States Army. He concludes that, charcoal is somewhat better than coke as a reducing agent. It has a higher electrical resistance, increases the furnace temperature, and decreases the power consumption per ton and the speed of production. An estimate of the cost of the raw materials for such electric smelting is as follows:
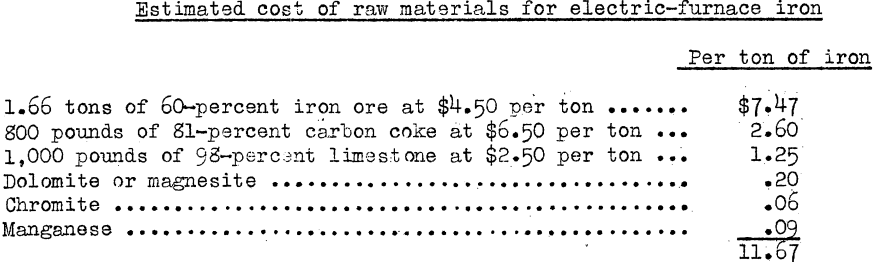
A report by Miller followed that by Hodge. His introduction states that it became apparent that the cost of producing pig iron in the Portland-Bonneville area would be much higher than the cost of the competitive blast-furnace iron at Ironton, near Provo, Utah; and that the feasibility of an electric-smelting enterprise could be determined only after detailed and careful study had been made of all items of cost.
In selecting a furnace there are only a few types to choose from, and only two of the many that have been tested have survived and been developed into industrial furnaces – the Swedish “Elektrometalls”, or shaft type, and the Norwegian “Spigerverk”, or pit type. The first of these has been operated since 1911, and the other since 1925; at present the shaft furnace is being used in three plants and the pit furnace in two plants. The Swedish furnace has been developed to use charcoal exclusively, although it has been adapted to use 65 percent charcoal and 35 percent coke. The Norwegian furnace was developed for coke only, but it can be adapted to use charcoal only. Miller concludes that under the conditions existing in the Portland area, the Spigerverk furnace is most suitable.
Section 7 of volume 2, 93 pages, in Miller’s report, covers the electric smelting of iron ore as tried and as it is being done throughout the world. The Dominion of Canada made electric iron in 1906 and demonstrated that, metallurgically, a sustained operation could be carried on. But the furnace was converted to produce ferro-alloys.
During 1907 furnace runs were made in Shasta County, Calif., but they wore attended with difficulties, technical and economical. In 1925 an unsuccessful attempt was made to resume smelting.
Iron Ore Smelting
Norway has extensive deposits of iron ore containing 30 to 36 percent iron, as well as large deposits of pyrite which, after the sulphur has been removed, will, yield 60 percent iron. Norway not only has an adequate limestone reserve and ample water power, but is well-supplied with raw materials for making iron and steel. Charcoal blast-furnaces were used for many years to reduce the iron ore, but since 1910 the metallurgists have persevered with the electric furnace, according to Christiansen. After many trials with a 6,000-kv.-a furnace in 1925 and one of 9,000 kv-a in 1927. Norway now produces electric pig iron at three places – Christiania Spigerwerk, 12,000 to 13,000 tons a year; Bremanger, 18,000 to 20,000 tons a year; and Hoyanger, 4,000 tons as a byproduct. The iron made in Norway is a quality pig and the brand “Norway Iron” is as good as ever it was during its 200 years of use at home and abroad.
In the electric reduction of iron ores the nature of the reducing agent is of the greatest importance, Christiansen says. With charcoal the process runs smoothly, even in closed furnaces. Coke presents difficulties.
The Electrometal furnace is a charcoal type that can stand some addition of coke at the expense of increasing its consumption of power and electrodes and with additional repair costs. The waste gas is utilized outside. The furnace is expensive to buy and maintain and can make only iron of low silicon content, generally below 1 percent Si.
The Tinfos furnace uses coke only as the reducing agent. It makes gray iron of high Si content. The byproduct gas has not been used so far.
The Spigerwerk furnace also burns coke and coke dust. Utilization of the gas is practically complete. A high content of fine material in the charge does not interfere with regular working of the furnace. The furnace makes irons of different quality, from pure white iron of low Si content to iron with 4 percent or more of Si. The furnace is simple, low in first cost and repairs, consumes 2,300 to 3,000 kw.-hr. per ., ton of iron, and is the only electric pig-iron furnace that by using cheap reducing agents (no charcoal), utilizes the gas and permits the making of iron of any desired quality. Another advantage is that furnace units can be made larger than for other types.
Manganese Smelting
The Greene Electric Furnace Co., Seattle, Wash., through Albert E. Greene, has shown us a copy of a report made to the War Department on the reduction of manganese silicate ore from the Olympic Mountains. The purpose of these tests was to make ferromanganese from an ore that averaged 33 percent Mn, 8.6 percent Fe, and 24 percent Si. It was concluded that a low-silicon ferromanganese could be made. Among the furnaces tested was one 36-inch Greene with two vertical electrodes and one Greene with two horizontal electrodes; the others were shop made. The feature of interest to readers of this information circular is that when finely ground manganese ore, coal, and lime are subjected to a relatively high temperature, globules of metallic ferromanganese are formed. The charge is then cooled, ground, and the metal separated by gravity concentration. The fine ferromanganese can be used as such or be remelted. This is termed “high- temperature, nonfusion reduction” in distinction to “low-temperature fusion reduction”, which consists in melting the alloy into a bath.
Consumption of fuel, flux, and power are approximately as follows per ton of ferromanganese in large-scale operation: Coal, 1 ton; limestone, 1-½ to 2 tons; energy, 2,000 kw.-hr. (reasonably hoped for); electrodes, minor. A basic (magnesite) hearth lining would be necessary for this process.
The nonfusion reduction process may possibly be adapted to the treatment of chromite.
Smelting Chromite
At Ohanga Lake, near the Canadian northern line in Ontario, the Chromium Mining & Smelting Corporation has chromite averaging 17 percent chromic oxide and is concentrating it to over 40 percent. The concentrate is railed to Sault Ste. Marie, where the company has three electric furnaces making ferrochromium and other products. One furnace (recently dismantled, but to be rebuilt) made ferrochromium-silicon, and a second makes low-carbon ferrochromium from the product of that furnace. The furnace making this ferrochromium differs from the others, in that the smelting is done in an enclosed crucible rather than out in the open. It is of 7-ton capacity, operates at 110 volts, and has an approximate rating of 1,200 kv-a.
With regard to chromite, Robert M. Keeney covered at length the electric smelting of chromium, tungsten, molybdenum, and vanadium ores in Transactions 24 of the American Electrochemical Society, 1914. These ores are not smelted at the mines, but after concentration there, the enriched product is sent to electric furnaces in the East and converted into ferroalloys. The temperatures required are so high that reduction in the blast furnace is not possible, nor in a reverberatory or crucible.
The following item on the electric reduction of tin is from The Metal Industry (London) for January 15, 1937:
An interesting example of the use of the electric furnace in the smelting of tin concentrates is the process used at Anneccy, the smelter of La Societe d Electro-Chimie et d’Electro-Metallurgie et des Acieries Electrique d’Ugine. The concentrates, which are generally of Bolivian origin, are mixed with calculated quantities of coke, lime, and other fluxes, and agglomerated by sintering with a hinder under a pressure of 400 kg/mm. The agglomerate is introduced into an electric furnace with a single electrode and conducting hearth. A slag of monosilicate degree is aimed at in the calculation of the furnace charge, and this will be fairly high in iron and tin since, owing to the effective nature of the second or slag-cleaning operation, it is not found necessary to attempt to produce a cleaner slag at the first smelting. In the first operation dust losses are small, the Lodge-Cottrell plant socuring 98 percent recovery.
The molten products collect on the hearth and are tapped at intervals into ingot moulds, where separation into two layers occurs. The upper layer is a cake of slag which is sent forward to the second smelting charge; the lower layer consists of substantially pure tin, which is suitable to be sent at once to the liquating and refining operations.
The slag is crushed, mixed with ferrosilicon, lime, and coke, and treated in a furnace exactly similar to that used for the preceding operation. The object of this second smelting is the production of a sesquisilicate slag substantially free from tin. The products of the fusion are collected by tapping into carbon-lined moulds, and in this case separate into three layers. At the top is a cake of substantially tin-free slag, next the ferro—silicon, which is now enriched in iron, and finally, at the bottom, molten tin suitable for liquating and refining. Tho two superior layers are removed as, soon as they have solidified, and the molten metal ladled away into moulds.
The daily output of 7 tons is stated to be approximately equivalent to the capacity of a reverberatory furnace with 200 square feet of hearth area. The chief advantages claimed for this method of smelting are low dust losses, capacity of reaching smelting temperature easily, and long life of refractories.
The operations of liquation and refining bring the iron content down to a final figure of from 0.02 to 0.04 percent.
Electric Furnace Manufacturers
Although we know the names and more or less about the manipulation of electric furnaces so widely used in melting ferro-alloys, gray iron, steel, gold, silver, and nonferrous metals and alloys, we know of only one electric furnace that is advertised as being suitable for smelting at small mines – the Wile furnace.
Wile Furnace
According to Electric Smelters, Inc., Central City, Colo.:
There have been over 93 successful installations of the Wile electric furnace to date. These installations have covered the treatment of a great variety of ores, concentrates, and metals. In addition to these installations, laboratory tests on thousands of ores have enabled a vast amount of accurate data to be compiled about the smelting of almost every type of known ore combination. Accurate estimates of costs of operation may be given if a complete analysis of the material to be treated is furnished, together with costs of power, if available, and with all information covering any potential fluxes, such as iron, or manganese ore, or limestone.
The Wile electric furnace is adapted to, and in many instances has been used successfully for, the smelting of gold and silver ores, concentrates, cyanide precipitates, tin concentrates, soft-metal drosses, lead and lead-silver ores, battery plates, copper scale, copper ores, manganese copper, and for the recovery of metallic values remaining in slags produced in ordinary fuel-fired furnaces, nickel and cobalt ores, sponges and scrap, iron ores, manganese ores, and for the manufacture of ferroalloys.
The firm’s folder states that the Wile furnace has many advantages, chief of which is control of the temperature. The furnace operates on the principle of heat generated through the resistance furnished to the path of the electric current in a molten-slag conductor. The electrodes are vertical and dip into the slag in the crucible from above and below it.
Above the crucible is the charging throat. The largest size of furnace yet developed has a daily capacity of 150 tons of ore. As to cost of operation, there are broad limits, naturally. In general, a furnace of medium capacity would consume 250 kw.-hr. per ton of charge, which means, at 1 cent per kilowatt-hour, $2.50 per ton. As to recoveries, it is said:
Where the ore or concentrate must be roasted before smelting, the electrically smelted product is a metal bullion carrying the values. Some matte is also formed, but this is returned to the roaster to he re-roasted, while the bullion is sent to a refinery. Costs of refining are small, at the most, and when charged back to each ton of ore, are trifling. Refineries make no deductions from the prices of the metals, making only nominal charges for the refining.
In practice the slag is malted first, and a deep bath is formed. The charge is fed on top of the slag, and the reduced metal or matte sinks to the bottom of the crucible and is tapped as desired. The slag is drawn off continuously. No air is introduced into the furnace and any dust created is collected by the colder charge within tho furnace. No bag-house is needed. It is reported that the slag seldom has to be re-treated.
The authors wrote Electric Smelters, Inc., for further information, and R. S. Wile, president, answered our questions to this effect:
The firm had just received an inquiry as to whether it would pay to smelt locally concentrate from a mill that produced 3 tons of concentrate per day. The reply stated that because of its situation, and other factors, a satisfactory return was to be expected from smelting at the mine.
Wile states that he has spent over 30 years in developing smelting in the electric furnace, and as far as he is aware nobody else has attempted to develop a furnace to smelt anything but steel, brass, and alloys.
The largest furnace that Electric Smelters has built is smelting 100 tons of iron ore in Norway (per day, presumably). This was erected in 1916 but is not mentioned in Millers report of 1936 cited later. The smallest furnace has a capacity of 750 pounds in 24- hours and is employed for reducing precipitates. All of the Wile furnaces are of the continuous type, except those built in 1916 for the Rothert Process Steel Co. in Seattle for reducing titaniforous iron ores to refined steel in one operation. (We learn on authority that this plant has been idle since 1926.) There is no objection to batch smelting or shutting down the electric furnace for periods, excepting the loss in heat units resulting from such practice.
In smelting copper ores, black copper or matte can be produced, depending upon the roast or condition of the copper in the charge. In smelting copper-bearing gold and silver concentrates with a heavy pyritic base, both bullion or matte are made. If there is a deficiency of copper as a collector, either lead in some form or copper can be added, but pyrite has been used, leaving some sulphur in the roasted product. In the latter case, this must be converted and in the presence of copper.
In smelting lead ores, unless there is enough lead bullion it is more economical to send it to an existing lead-softening plant, which will do the work more satisfactorily than could a small auxiliary installation.
The Wile furnace is unable to smelt zinc ores. Zinc-bearing concentrates can be smelted after the sulphur has been roasted off.
Concentrates are not briquetted before smelting, and they may be charged as lump, sinter, or after being roasted. The action in the furnace is quiet and there is no great loss of dust. An expansion chamber is provided with the furnace for catching dust and to prevent.an explosion.
Upon learning that Wile furnaces had been installed in Colorado, California, and North Carolina, the authors wrote to the persons whose names had been given us, but the only reply received came from P. W. Royer, engineer for the Kelly Gold and Silver Mines, Red Mountain, Calif. He stated that the furnace was rated at 1,000 pounds daily capacity and was being used experimentally. The inventor claimed that it would smelt 1 ton of ore, consume 200 to 400 kw.-hr. and produce a temperature of 3,500°. Power is available at Red Mountain at 0.5 cent per kilowatt-hour, and the electric furnace will be given a fair trial; also, if it is successful, some operating details will be available at a later date.
Three electrometallurgists of the United States Bureau of Mines saw this furnace at Red Mountain at the time the heat was turned on for the first time. The purpose of this furnace is to smelt copper-silicate ore with silver ore. The furnace consists of a chamber or hearth 24 inches wide and 27 inches high surrounded by 9 inches of rammed magnesite. At the bottom center is a 3-inch graphite electrode, and at two top sides are 1-½-inch electrodes. There is a metal taphole near the bottom and a slag taphole two-thirds the distance from the bottom. The principle of operation is to keep the hopper shove the furnace and the furnace chamber full at all times, tapping slag and metal intermittently. The furnace power was three 5-kw. transformers, which would appear to be incapable of supplying enough energy to reduce the charge.
It was reported that a Wile furnace is used in Deloro, Ontario, for melting alloys, but none is used for smelting ore at this place.
According to Wile, a 15-ton furnace was installed and has been operated since September 1934 at the Russell Gulch Mining Co. property, Central City, Colo. Wile owns a quarter interest in the equipment.
Although it was reported by others to be idle during December 1936 it has smelted concentrates containing about 40 percent iron, 50 percent sulphur, some insoluble matter, a little copper, and 1-½ ounces each of gold and silver. Some results but no details of manipulation of this furnace were given in an article by Dale F. Underwood, now superintendent for the United Gold Mines, Sunshine (via Boulder), Colo., in “The Mining Mountaineer, Mountain States Mineral Age”, Denver, for March 1936 and what follows has been abstracted therefrom:
No matter where the mine is situated, the cost of handling a pyrite concentrate in this manner shipping to smelter is expensive and burdensome and, more frequently than not, unprofitable.
The mine owner then asks himself the question “Why cannot I do as does the custom smelter? Smelt my own concentrates”, and he begins to look around for a furnace which will do this very thing.
Underwood then discusses furnaces generally, including the economics, and says:
The electric smelting furnace is limited to the slag-resistance type, on account of the intense heat generated by the arc type, which is used for steel manufacture. With the slag-resistance type, the operator enjoys a quiet heat, with no blast, the slag being in a molten condition-beneath the ore charge; and the ascending heat, instead of being dissipated through a roof, is absorbed in the descending ore charge, with the bulk of the heat supplied to the furnace utilized in the reduction of the ore. The charge condenses any of the values, which may be volatilized, thereby eliminating expensive outside devices for catching these values. Often, the slag may be so constituted on account of the wide temperature possibilities, that a strong refining action may be had as the fine particles of metal filter through the deep slag resistance on their way to the bottom to be tapped when formed in sufficient amount.
The following figures compare the returns and costs of 24 tons of concentrates sent to a custom smelter and 24 tons smelted locally in the Wile furnace. The concentrates assayed 2.07 ounces of gold, 2.30 ounces of silver, and 1.85 percent copper.
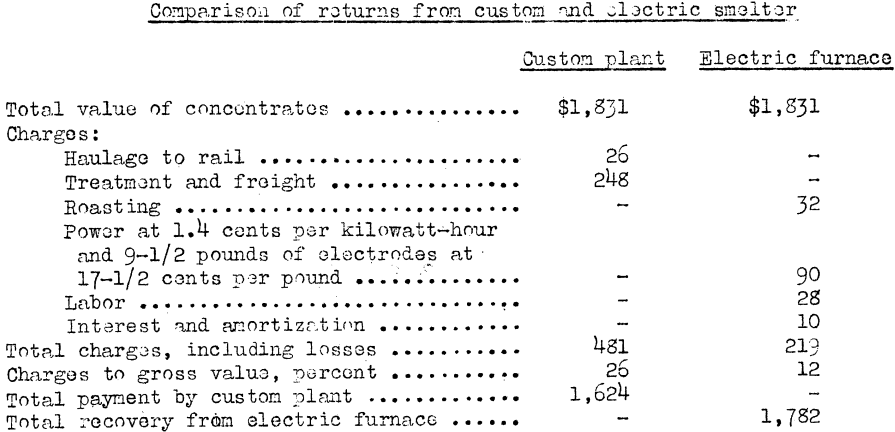
Underwood states that roasting is necessary for local smelting, the power charge has been reduced, and electric power is delivered regardless of weather and traffic conditions.
We have been informed that Electric Smelters, Inc., was trying to get permission to raise money to erect a 150-ton plant just outside Denver and proposed to do custom smelting. As such operations have not been carried on there for years, the possibilities for electric smelting in this center would appear to be rather problematical.
Smelting Furnaces
The Bureau of Mines asked the heads of well-known makers of electric furnaces that are used extensively in molting metals and alloys what they know of the electric smelting of ores, and their replies contained the following information:
- There is nothing theoretically impossible about smelting any of the metallic-ores, though experiments on lead and zinc ores so far have failed; at least, not one electric furnace is at work on a plant scale.
Our firm built a 300-kw. electric furnace for copper ore at a small mine in a difficult region in the West, but it was abandoned although a considerable quantity of marketable copper was produced. This experience was rather favorable and showed that oxygen-free copper is possible from the electric furnace. - The firm does not know of any smelting of copper, gold, silver, lead, or zinc ores in electric furnaces at the present time. They would refer you to the papers of E. A. J. Fitzgerald, E. T. Snyder (in 1911), and others (before and later) in the Transactions of the Electrochemical Society. These writers describe furnaces that at the time were giving apparent satisfaction but which later were discarded.
- As far as this firm knows, there is no reason why a workable electric smelting furnace in small sizes cannot be made. However, so far we have not found a case where its use would be within the range of practical economics. There is need for the dissemination of fundamental information on smelting processes among the many small organizations that are interested in the problem.
There is a lack of understanding of the difference between the reduction of an ore and the melting of a metal, this being the cause of much stumbling along the metallurgical path that untrained men seek to follow. Also, there appears to be a fixed belief by many men that electric current does something in its passage through a material besides produce heat, and the amount of money that is being spent here and there by laymen because of that idea is surprising. It may be true, but if so it is beyond our knowledge of molecular and atomic physics. - Our attitude with regard to the use of electric furnaces for smelting ores is that each case usually involves special study and consideration and that, generally speaking, there is no standard furnace available for such work. The proper furnace and its design would have to be determined by a study of the ores to be treated, local conditions, economics, and other factors. We have not had any experience in smelting ores, our equipment having been practically limited to melting metals and alloys. We have experimental facilities and possibly could try smelting ores, but each would require individual study.
- This firm of wide electric and manufacturing facilities does not build electric furnaces for smelting ores, but it does make accessories for furnaces. It has no information on the electric reduction of ores.
- We frequently receive inquiries from individuals who are interested in smelting or melting operations and who feel that if a furnace is electric it is something to conjure with. As a, rule, these inquiries are not on a sound basis and the efficacy of shaft-type furnaces may be questioned. Electric smelting furnaces for ores have not been popular in the United States, and even in Europe there are few that give good practical results. We have had some experience with a shaft-type furnace, but it did not give satisfaction and was replaced by a standard, open-top, three-phase, arc-resistance furnace for the production of ferrosilicon. Many difficulties are encountered in a shaft-type reduction unit and the most important item is the cost of energy.
- With cheap electric power the furnace to be described might be used on ores, but a well-known firm with an interest in it questions that it has advantage over the standard types in smelting. On December 22, 1931, United States Patent 1,837,696 was issued to Sydney T. Wiles (not Wile) for the electrothermic reduction of iron ores. On October 6, 1936 this was reissued as Reissue 20,128 to Wiles (deceased) and assigned to the Buffalo Electric Furnace Corporation. Drawings show cross-section and other views of a revolving electric furnace. The cylindrical shell is lined with firebrick, outside of which is magnesite or other refractory. On both sides of the shell is a hopper, into which is charged 50-mesh or finer iron ore, charcoal, and limestone. The hopper feeds a screw-conveyor, which works within a short metal tube and forces the charge to the interior of the furnace through hollow electrodes. Surrounding each electrode at its point of exit from the furnace is a cooling device, which is also used to remove waste and possibly explosive gases from the furnace, a fan being part of the equipment. In operation, the furnace is brought to the required temperature and the powdered charge fed into the furnace, which is rotated. When enough material has been fed, and all is molten, the tap-holes are opened and the metal and slag run out.
Conclusions and Recommendations
Consideration of the foregoing discussions lead to the following conclusions regarding the electric smelting of ores and concentrates:
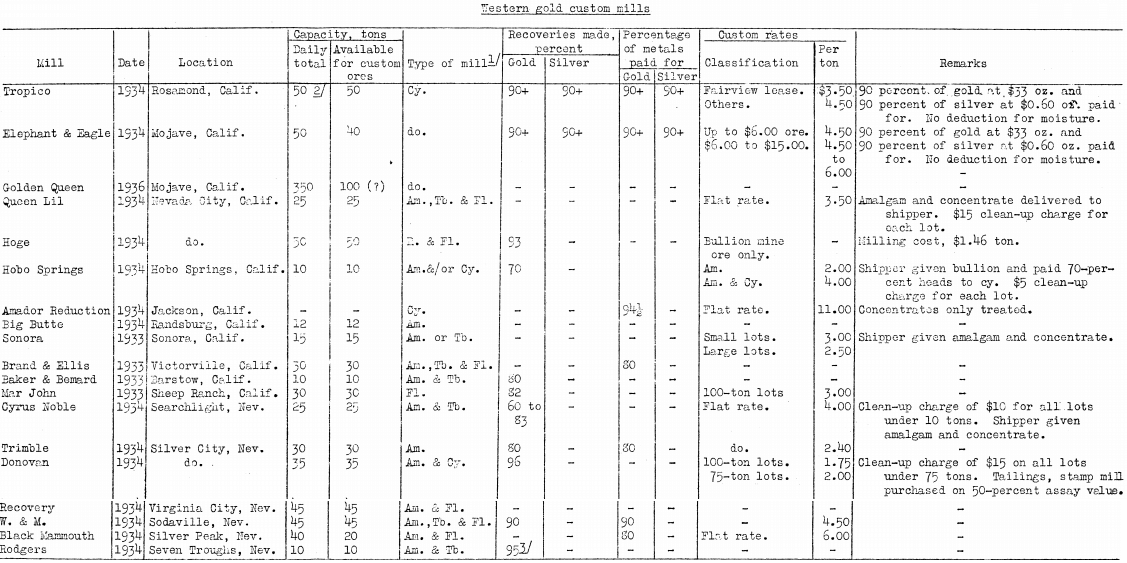
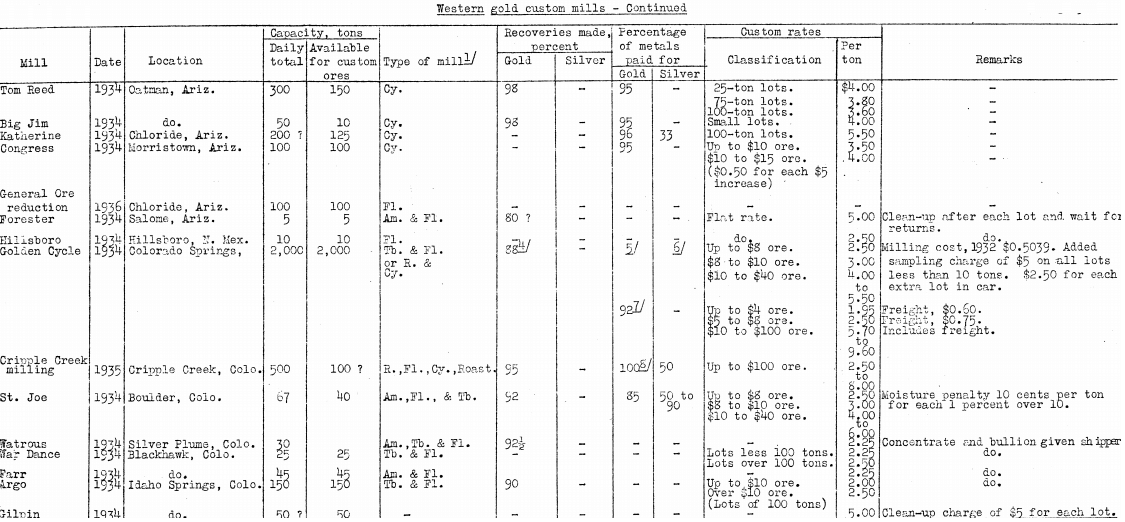
- Before a decision is made to use electric-furnaces for reduction, it would be advisable to ascertain conditions at the nearest suitable custom mills and smelters. The authors reprint the table, of custom plants (brought to date) from a previous Bureau publication, and have compiled a list of smelters from a reliable source.
- As more or less continuous (day and night) operation of any smelter is necessary for smooth end economical results, the mine owner should be certain that he has a large tonnage of ore developed before he attempts to install smelting equipment. We recently learned of such an installation a blast furnace heated with oil, which did not work.
- The smelting of an ore differs considerably from the melting of a metal. The latter needs mainly heat whereas the former requires heat, and fluxes. In reducing a copper ore, the intermediate product is matte, which is then blown to blister metal and this finally has to be refined electrolytically; in reducing a lead ore, the intermediate product is lead bullion which has to be cupelled; the treatment of zinc-ore is difficult and involved; concentrates are more or less troublesome.
- Although the authors have tried to obtain information on the current smelting of ores in electric furnaces, apart from what has been and is being done in Norway, Sweden, and Finland, we have been unable to procure any definite information regarding operation or results. In those countries fuel is scarce but electric power is cheap.
Following are lists of reliable and suitably situated smelters and custom mills for any type of ore in North America, complete to the end of 1936:



With regard to the mills and smelters listed, the authors learn on reliable authority and state without any prejudice that because there is only one smelter in the Leadville region, Colorado, a number of small mining companies have become interested in finding a cheaper and better means of having their ores smelted; therefore, the experimental electric furnace near Central City has created some interest in Colorado. But smelting is not the only method of treating ores, because at Colorado Springs is a 2,000-ton mill that can and does treat a variety of precious-metal and base-metal bearing ores by flotation, cyanidation, or both processes. Freight rates are a factor, of course, but surely the smelter at Leadville or the mill at Colorado Springs makes satisfactory payments on ores sent for treatment.
If the mine owner or mill operator wishes advance information on the charges imposed at smelters, he should procure from United States Bureau of Mines its recent report by Gardner and Alisman. This was written especially for the operators of small gold and silver mines. Gold and silver ores or concentrates are smelted at both copper and lead smelters; one of these base metals is required to collect the precious metals during the process of smelting. The art of smelting, effects of impurities, sampling and assaying, freight and trucking rates, a list of copper and lead smelters in the West, and the schedules that give the payments, deductions and penalties, and treatment charges are all given in detail with examples of calculations. Every mine operator who is shipping or contemplates shipping ores and concentrates to a smelter should have a copy of that report.