Table of Contents
One of the major technological problems associated with beneficiation of complex, finely disseminated ores is the generation of large quantities of fine minerals less than 10 µm in size. Fines are generally refractory to conventional low-cost beneficiation methods, and failure to recover this fraction can represent a significant, permanent loss of valuable minerals. In addition, fines in conventional flotation circuits may be nonselectively entrained, leading to lower grades. Fines also form slime coatings on other minerals, which prevents adequate collector/mineral surface interactions, and results in poor recoveries and/or low grades. They may also report to the tailings where they pose problems in water clarification or in meeting pollutant discharge requirements.
For a number of years, it has been recognized by Slater and co-workers (1969), Kitchener (1972), and Attia and Kitchener (1975), that one of the more promising methods to recover fines might be their selective flocculation with polymeric materials containing specific chelating groups. Although flocculants are widely used in water clarification, their more general use in mineral recovery has not been realized, and considerably more research is required in this area. For this reason, the Bureau of Mines has been conducting laboratory studies on the flocculation of a number of minerals with polymers (crosslinked starches) containing various chelating groups with the goal of identifying promising flocculants and determining the conditions required to obtain mineral selectivity. Previously, Termes and co-workers (1982) reported on the selective flocculation and separation of bornite from quartz using insoluble crosslinked starch xanthate (ISX). In the present paper, the flocculation of oxide and hydroxide mineral fines of hematite, goethite, chromite, cuprite, manganite, pyrolusite, and cassiterite with chelating crosslinked starches, including ISX, is reported. Selective flocculation of hematite, chromite, and cuprite from mixtures with quartz has been attained with some of the starches, and is discussed in detail for hematite.
The crosslinked starches (CLS) are epichlorohydrin crosslinked materials which can be represented as
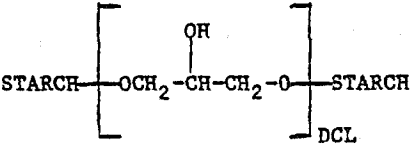
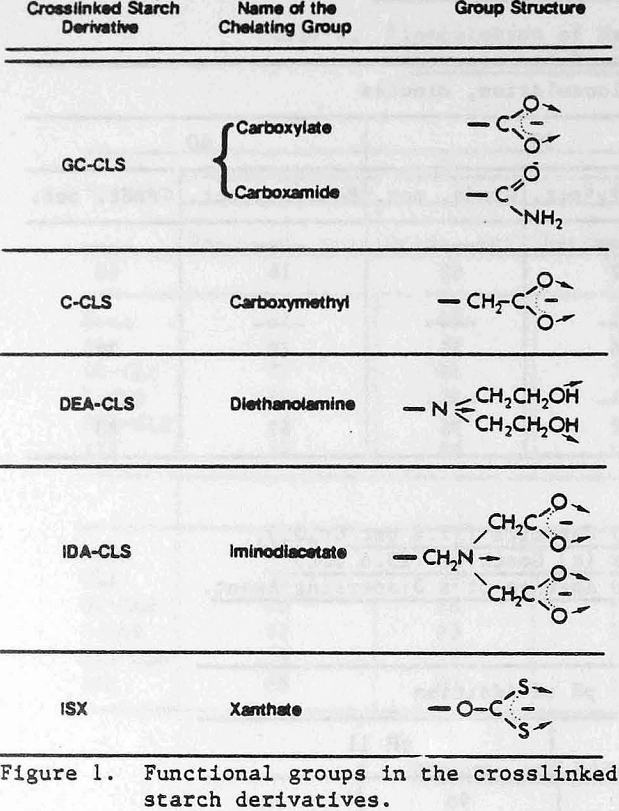
where DCL is the degree of cross linking. The derivatives contain the following chelating groups: (1) carboxyl, (2) iminodiacetic acid, (3) diethanolamine, and (4) xanthate. The structures of the chelating groups in the cross-linked starch derivatives reported here are shown in Figure 1. The arrows indicate the donor atoms that may be involved in bonding. The starches were synthesized by Wing and co-workers at the U.S. Department of Agriculture, Northern Regional Research Center, Peoria, Illinois, for use in heavy metal removal from contaminated waters (references below). Only ISX is commercially available at the present time. The other starches were research materials provided by the U.S. Department of Agriculture.
Two types of carboxyl-containing crosslinked starches were investigated. One of them is a grafted carboxyl derivative (GC-CLS). It contains a mixture of carboxamide and carboxylate groups formed by the saponification of acrylo-nitrile, which had been grafted previously to the starch. The other carboxyl-containing starch (C-CLS) is a carboxymethyl derivative. The preparation and use of GC-CLS and C-CLS are described by Rayford, Wing, and Doane (1979).
The synthesis and reactivity of the iminodiacetic acid (IDA-CLS) and the diethanolamine (DEA-CLS) crosslinked starches were reported by Rayford and Wing (1979, 1980). These two materials were synthesized from a crosslinked starch containing 3-chloro-2-hydroxypropyl ether groups as the intermediate. Upon reaction with the sodium salt of iminodiacetic acid or with di-ethanolamine this intermediate yielded IDA-CLS and DEA-CLS, respectively.
Experimental
Reagents: Unless otherwise specified, all chemicals used were reagent grade and all water was distilled and deionized. A commercial highly crosslinked starch (Vulca 90 from the National Starch and Chemical Company) was used both to prepare ISX and in experiments in which unmodified CLS was investigated as a flocculant. The search derivatives GC-CLS, C-CLS, IDA-CLS, and DEA-CLS were obtained from the U.S. Department of Agriculture. The ISX was prepared at Avondale using the method described by Wing and Doane (1977).
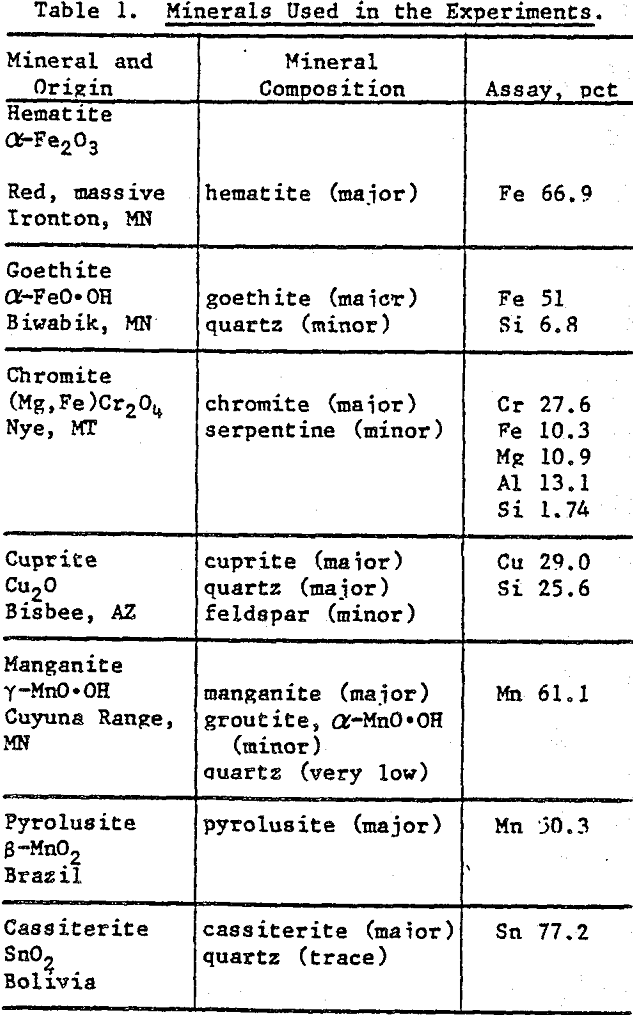
Mineral Preparation: Mineral specimens were obtained from Ward’s Natural Science Establishment, New York. Hand-picked crystals were ground to -400 mesh with an agate mortar and pestle. X-ray diffraction and wet chemical analyses were used to characterize the minerals. Table 1 summarizes the analytical data.
Flocculation Experiments: Selective flocculation studies were done using a binary mixture of the mineral with quartz in a preselected weight ratio (see Results section) to a total weight of 3 g. The mixtures were placed in 250 ml glass-stoppered cylinders after which they were filled with water to about the 210 ml mark and mixed in an ultrasonic bath for 5 minutes. Adjustments in pH with HCl or NaOH solutions (1.0 or 0.1 M, as needed), or addition of a dispersing agent, were done before mixing. The flocculant was placed in 40 ml of water and sonicated for 2 minutes immediately before addition to the mineral pulp. The pulp and flocculant were mixed for one minute by inverting the closed cylinder several times and then allowing the cylinders to stand for 5 minutes. The mixture was then transferred to a column elutriator (Fig. 2). The use of the elutriator provided a standard, reproducible method to separate the unflocculated material from the flocculated material. For the separation, Stoke’s law (Rose, 1954) was applied to determine the upward flow rate necessary to retain in the column all material larger than 40 µm (hence flocculated) and to elutriate all material smaller than 40 µm (unflocculated). Two flow rates were used: an initial flow rate of 225 cc/min and a final rate between 420 and 500 cc/min. The volume of water passed at the lower rate was 2 liters while the volume passed at the higher rates was 3 liters. Elutriating tins was 15 to 16 minutes. Following elutriation both the elutriated and floe fractions were collected by conventional vacuum filtration.
Other Measurements: Zeta potentials were measured with a Zeta-Meter. The values reported at each pH represent the average for 10 particles. Photomicrographs were taken with AMRAY Model 1400 SEM using the energy dispersive mode for element mapping.
Results
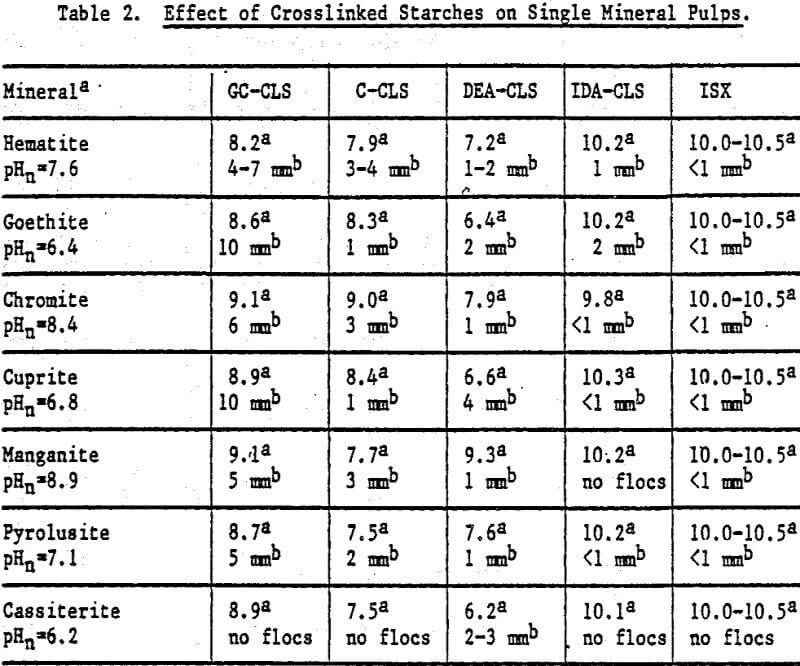
Flocculation Response: An estimate of the ability of each starch to flocculate the minerals was obtained by adding the starch to the mineral pulp at natural pH (pHn). The observations, including pHn and pH after starch addition where flocculation was observed, are summarized m Table 2. For most minerals, the largest and strongest floes were produced by GC-CLS. The floes, which can be redispersed to allow the release of unflocculated material, tend to reform readily. Of the starch derivatives tested, only DEA-CLS flocculated quartz to a slight extent.
Starch selectivity was evaluated based upon the recovery and grade of metal oxide or hydroxide mineral recovered from a quartz mixture, where postflocculation separation was done by elutriation. Grade refers to the weight percent of the metal oxide or hydroxide mineral in the flocs.
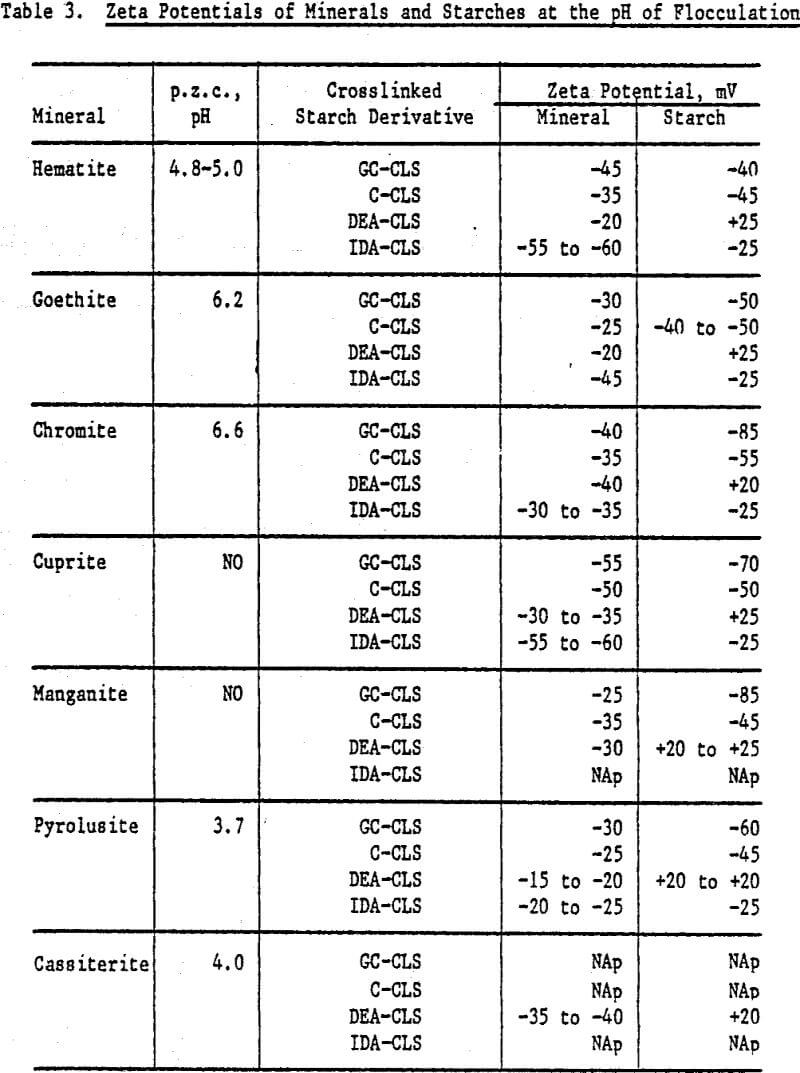
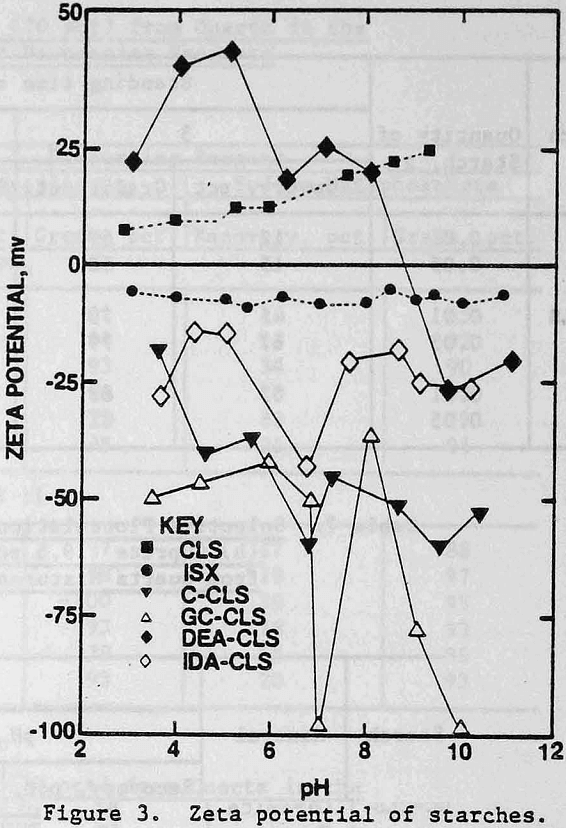
Zeta Potentials: Negative zeta potentials were found for ISX, GC-CLS, C-CLS, and IDA-CLS over the pH range of 3 to 11, in contrast to the positive values for CLS. The DEA derivative exhibited a point of zero charge (p.z.c.) at pH 8.7 and negative zeta potentials at pH values above the p.z.c. From Figure 3, it is seen that the zeta potentials of GC-CLS and C-CLS are more negative than the zeta potentials of IDA-CLS and ISX, particularly in the vicinity of neutral pH and above pH 9.
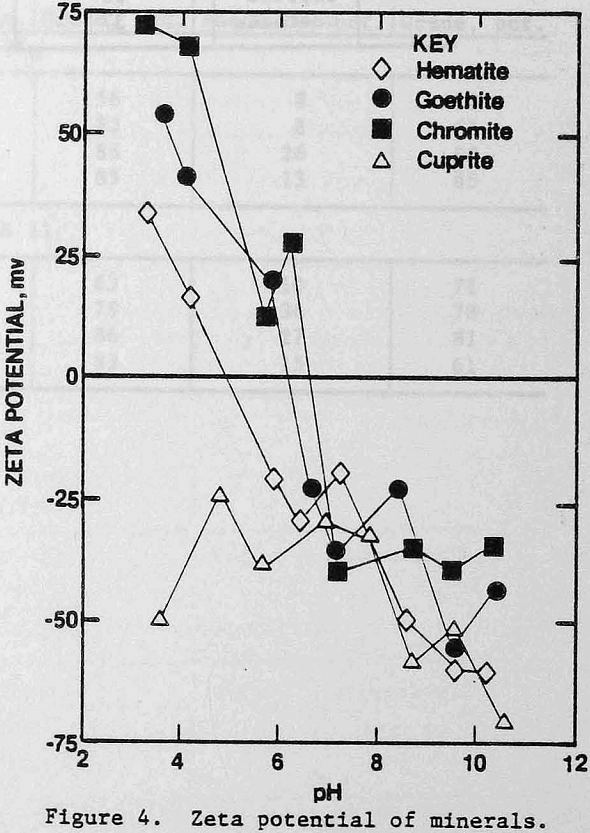
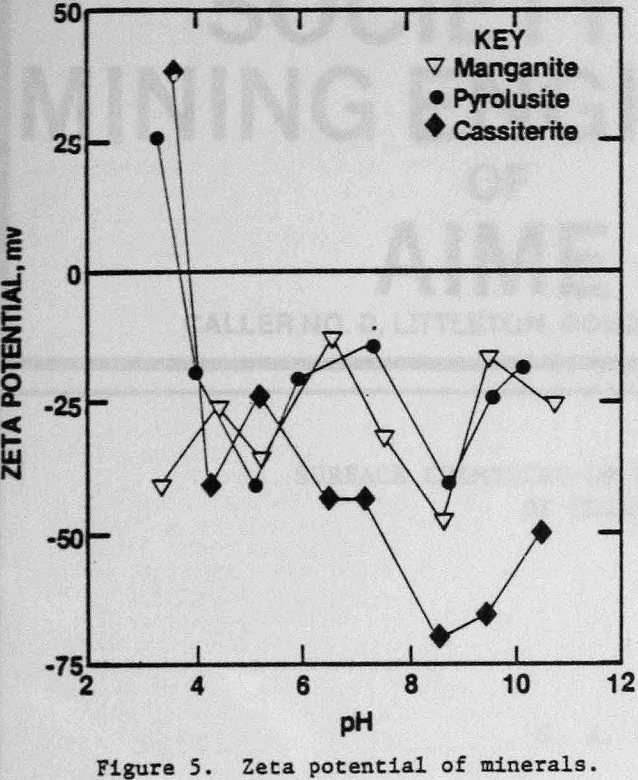
The zeta potentials of the minerals are shown in Figures 4 and 5 and Table 3 lists the zeta potentials for the minerals and the starches at the pH values at which flocculation was observed. Each mineral, as well as GC-CLS, C-CLS, and IDA-CLS, is negatively charged. The magnitudes of the repulsive electrostatic forces are also sufficiently large (Healy, 1979) to suggest that the interaction of these starches with the minerals most probably involves chemisorption through the chelating group similar to the behavior of ISX with sulfide minerals (Termes, Wilfong and Richardson, 1982). DEA-CLS is positively charged and adsorption may occur by electrostatic attraction with an additional contribution from chemisorption.
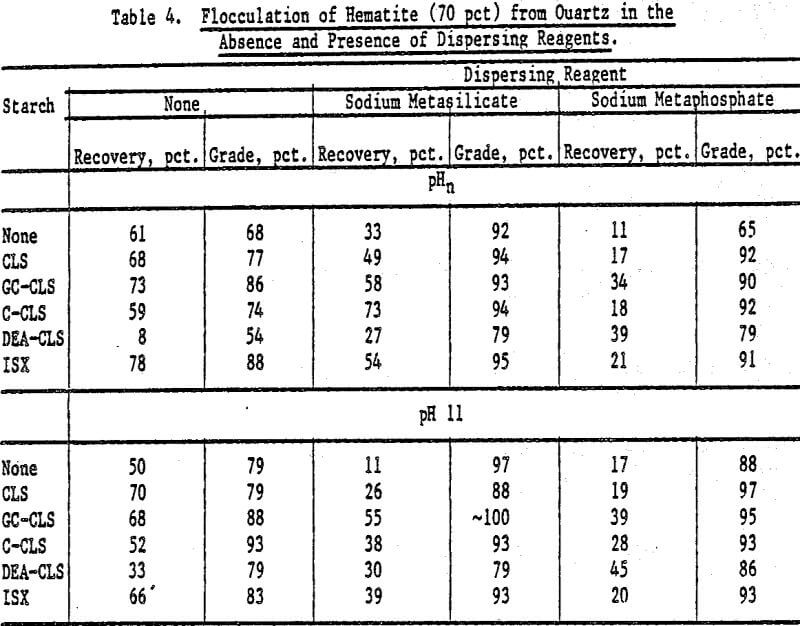
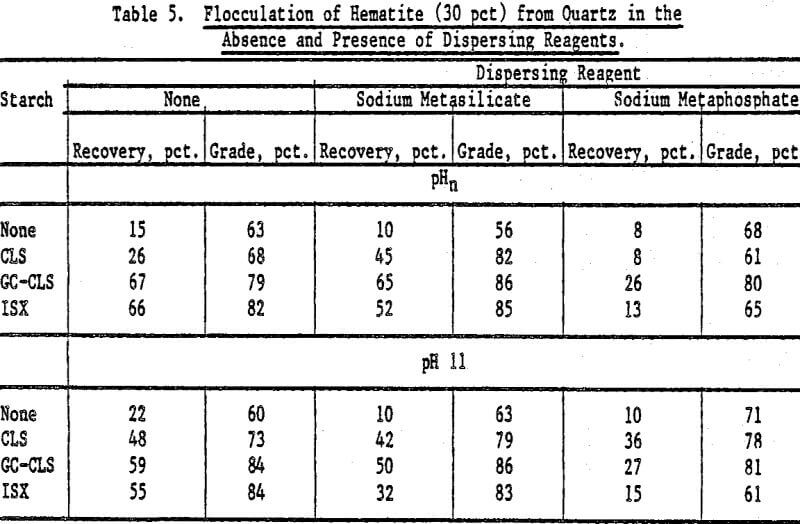
Selective Flocculation of Metal Oxide and Hydroxide Minerals from Quartz: Mixtures of hematite and quartz were prepared in the following proportions by weight: (1) 70 pct hematite and 30 pct quartz, and (2) 30 pct hematite and 70 pct quartz. The pulps contained 3 g of mixture in 0.250 liter of fluid; the starches (0.05 g unless otherwise stated) were added at pHn and at pH 11 (adjusted with NaOH) in the absence and in the presence of a dispersing reagent. The concentration of dispersing agent in the pulp was 1 x 10 -4 mole/L. The results are expressed in terms of hematite recovery and grade.
The results for mixtures containing 70 pct hematite and 30 pct quartz in the absence and presence of dispersing agents are presented in Table 4. For GC-CLS, C-CLS, and ISX, recoveries were, in general, higher when these starches were added at pHn than at pH 11. Sodium meta-silicate was a better dispersing agent than the metaphosphate, except in the case of DEA-CLS. In the latter case, higher recoveries and grades were obtained in the presence of sodium metaphosphate at pH 11. The results of flocculation experiments for the 30 pct hematite mixtures using CLS, GC-CLS, and ISX are given in Table 5. For the 70 pct mixtures, it can also be seen that recoveries were higher when GC-CLS and ISX were added at pHn. In these tests, as in the 70 pct hematite tests, sodium metaphosphate was found to be a poorer dispersing reagent than the metasilicate.
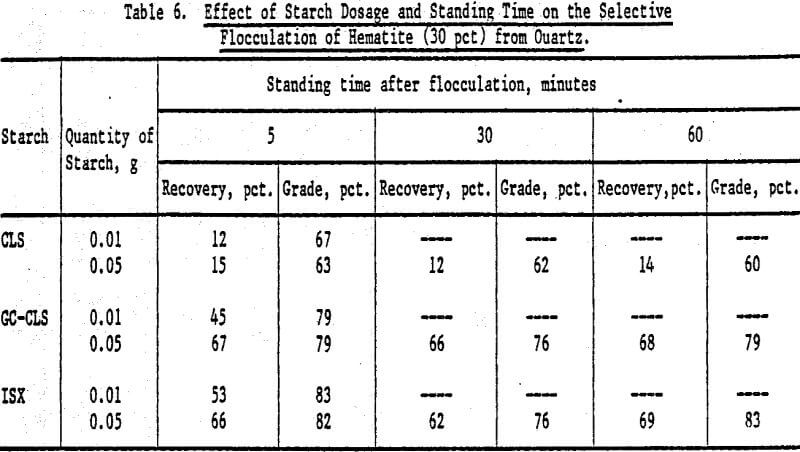
An increase in the starch dosage produced an increase in recovery, but grades were not significantly altered (Table 6). There was no marked effect on recoveries and grades as a function of time elapsed between flocculation and elutriation.
In the selective flocculation of hematite and other metal oxides, it was observed that GC-CLS formed large flocs within 2 minutes of mixing in the pulp, but no further floe growth was detected. ISX floes were smaller and grew more slowly. The flocs formed by ISX broke more readily during elutriation than did the floes formed by GC-CLS. Many small ISX-metal oxide flocs were carried away with the nonflocculated material, but were seen to re-form later into larger flocs.
When mixing of the flocculants was done with a mechanical stirrer, increasing the stirring rate did not appear to affect the amount of solids-recovered with GC-CLS as flocculant, but there, was a decrease in the amount of solids recovered with ISX, probably due to breakage of floes by the higher stirring rates.
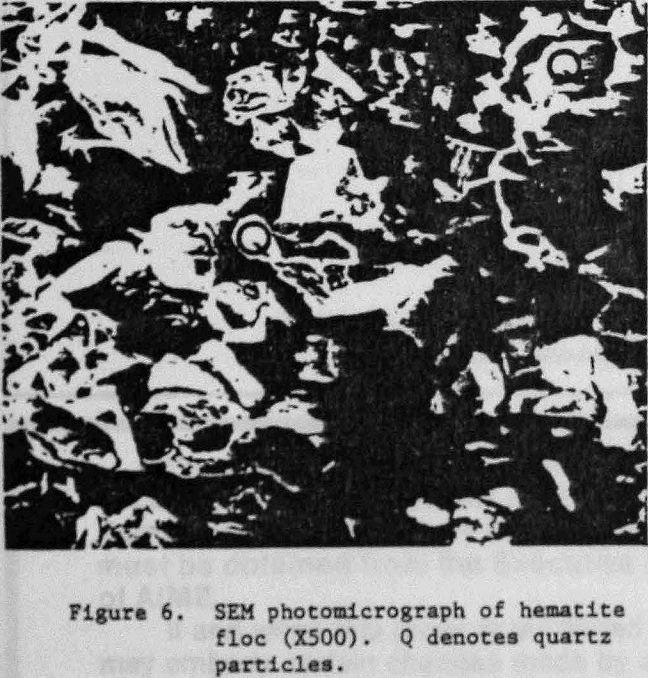
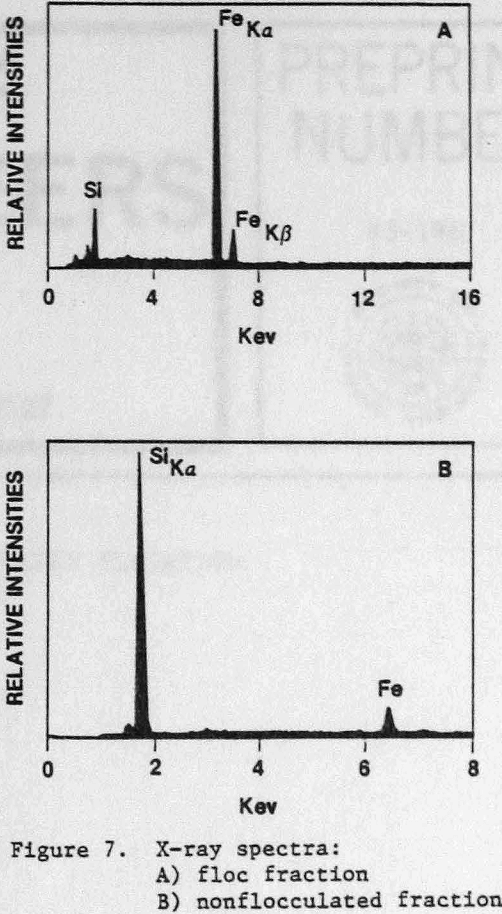
An SEM photomicrograph of a hematite floc is shown in Figure 5 where the hematite had been selectively flocculated from quartz with GC-CLS. After separation by elutriation, the floc fraction was recovered by filtration. The flocs were resuspended in water, and a few small flocs were placed on top of an SEM holder where they re-formed into larger floes; the water was then allowed to evaporate. The X-ray spectrum of the floc fraction showed that this fraction was enriched., in Fe (Fig. 7a), whereas the nonflocculated fraction was Si-enriched (Fig. 7b). Mapping of, Fe and Si established that the quartz was present in the flocs as isolated, entrapped particles.
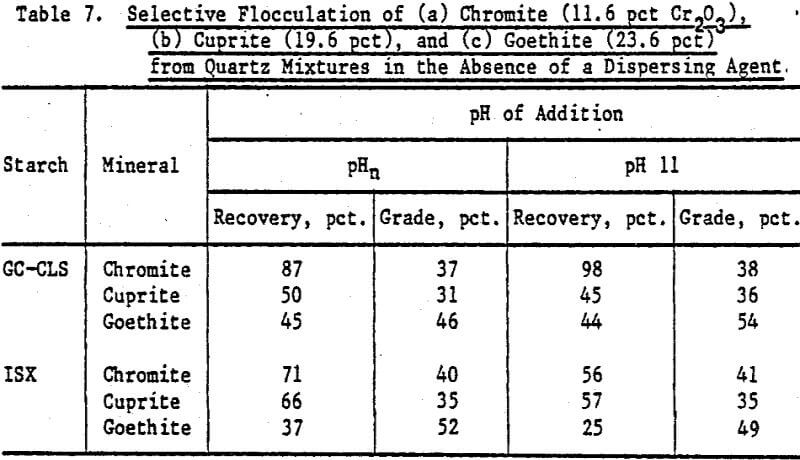
Table 7 presents results of flocculation experiments involving chromite/quartz, cuprite/ quartz, and goethite/quartz mixtures with GC-CLS and ISX as flocculant in the absence of a dispersing agent. The highest recovery (98 pct) was obtained for chromite with GC-CLS added at pH 11. The Cr2O3 content (grade) in these flocs is about 37 pct. For ISX-flocculated chromite the grades approach the Cr2O3 content in the chromite mineral (40.3 pct), indicating a slightly better selectivity of ISX towards chromite; however, the recoveries with ISX are lower than with GC-CLS. There is about a 1.7-fold increase in cuprite content in the flocs produced by GC-CLS and ISX when compared with the original mixture, but recoveries were higher with ISX. In the case of goethite, there is about a 2-fold increase in goethite content in the floes compared to the original mixture, but the recoveries were not high, especially for goethite flocculated with ISX.
Discussion
Crosslinked starch derivatives containing carboxyl, diethanolamine, iminodiacetic acid, and xanthate groups have been found to flocculate metal oxide and hydroxide mineral fines. It is extremely difficult to establish experimentally that a covalent bond forms between the chelating group in these large polymers and the mineral because of the low density of surface bonds. However, the known reactivity of the chelating groups towards metal ions and minerals (which is well known in the case of xanthate and carboxyl groups), coupled with the large electrostatic repulsive forces indicated by the zeta potential measurements, suggests that covalent bond formation contributes to the flocculation mechanism for GC-CLS, C-CLS, IDA-CLS, and ISX. In the case of CLS and DEA-CLS, the reaction mechanism leading to flocculation may be even more complex, with contributions from both electrostatic and chemical forces.
Carboxyl groups are well-known to chemisorb strongly on oxide mineral surfaces (see, for example, Fuerstenau and Palmer, 1976). The fact that the carboxyl-containing crosslinked starches formed rather large flocs with almost all of the minerals reported here, suggests that these derivatives may not be highly specific, although they are selective towards a metal oxide when present in quartz mixtures.
The higher grades of chromite in flocs separated from quartz using ISX (Table 7) when compared to hematite-ISX flocs (Table 5) may be indicative of a stronger interaction of ISX with chromite than with hematite, which in turn may be possibly related to the relative surface metal-sulfur bond strength. Note that the chromite grade in the flocs (Table 7) is expressed as a percent of the Cr2O3 content. In the chromite sample, the Cr2O3 content is 40.3 pct. A grade of 37 pct Cr2O3 in the floc fraction is thus 92 pct of the theoretical maximum grade of chromite.
The iminodiacetate group (Figure 1) is particularly interesting because not only the oxygens, but also the nitrogen are donor atoms. Flotation collectors containing oxygen and nitrogen donors have proved to be very selective, at least on a laboratory scale. Examples are the use of hydroxamates in the flotation of synthetic hematite (Raghavan, and Fuerstenau, 1975) and of oximes for copper minerals (Nagarai and Somasundaran, 1981). Work is currently being done to evalute IDA-CLS as a selective flocculant. Preliminary work has indicated some selectivity towards sphalerite and zincite from quartz and of galena from sphalerite.
Summary
Flocculation of oxide and hydroxide mineral fines by a variety of crosslinked starch derivatives has been studied. The presence of a chelating group on the starch results in enhanced selective flocculation. Covalent bond formation between surface metal component(s) and the chelating group appears to be involved in most of the cases.
Selective flocculation of hematite from mixtures with quartz has been attained. Recoveries and grades are dependent on the functionality of the starch as well as on pH and the type of dispersing reagent.
Acknowledgments
The authors express their appreciation to Dr. Robert E. Wing of the U.S. Department of Agriculture, Northern Regional Research Center, Peoria, Illinois, for providing us with samples of GC-CLS, C-CLS, DEA-CLS, and IDA-CLS and for helpful discussions, and to Mr. J. A. Riehl, Chemist, Avondale Research Center, for making available zeta potential data.