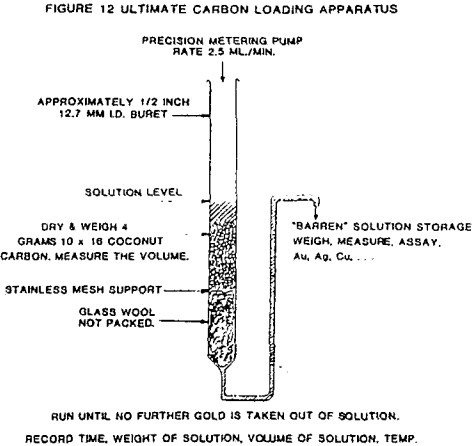
The procedures and tests descriptions are given for the ultimate gold carbon loading tests and the dynamic gold carbon loading tests.
A 4.5 parts per million gold solution was prepared by dissolving fine (-200 mesh) gold powder in a sodium cyanide solution made with deionized water at pH 10.5 (lime adjusted). This gold feed solution was fed by a metering pump at a rate of 2.5 ml/min. to each carbon loading column. The feed solution was analyzed for gold, silver and copper to monitor potential interference to gold loading.
A gold/cyanide solution was prepared in the same manner as previously stated. The solutions of ionic contaiminant were prepared in deionized water with appropriate amounts of sodium cyanide to dissolve the silver, copper, etc. powders. The gold/cyanide solution was then mixed with the contaminant solution.
Ultimate Loading and Ionic Contaminant Effect on Loading Rates
Activated coconut carbon prepared by various manufacturers is routinely used successfully by Mountain States Research and Development and by most carbon-process gold operators to recover gold from leach solutions and slurries.
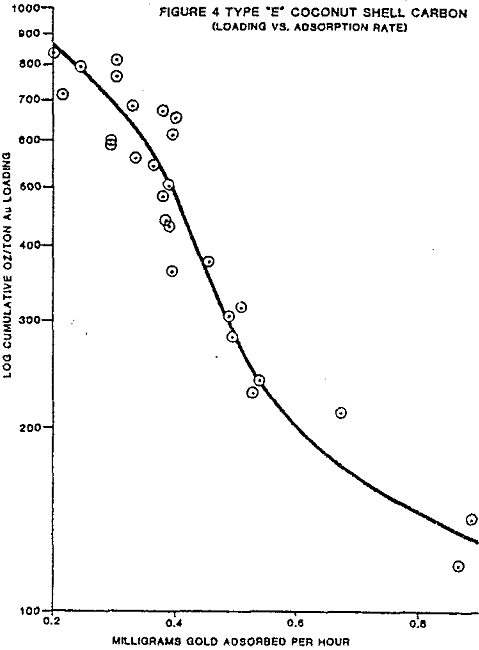
The test work on side by side gold loading comparison, of different manufacturers carbons has shown two carbons, type A and E, are far superior in total ultimate loading and the rate of gold adsorption.
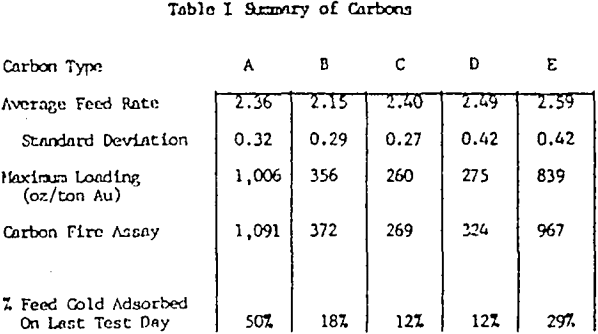
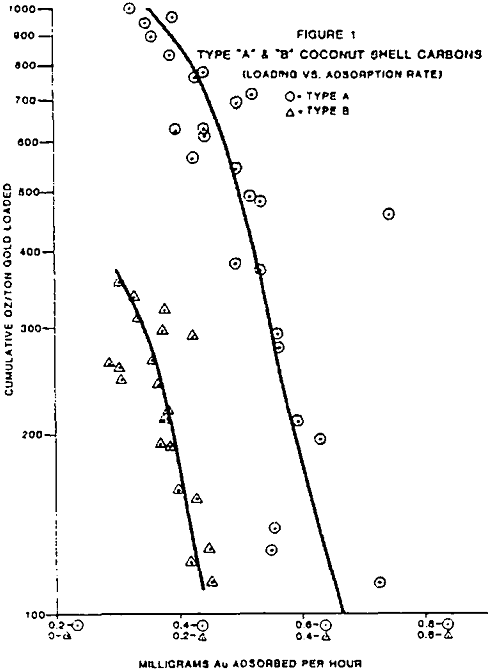
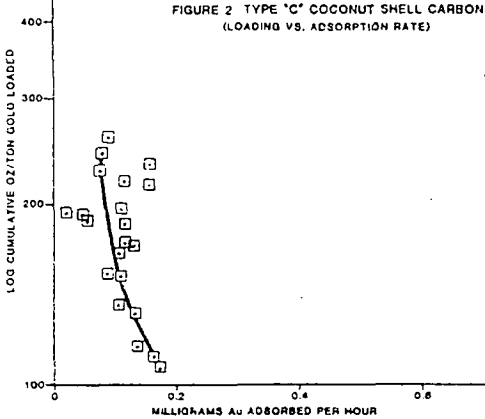
Pilot and commercial plant experience in the operation of carbon column and CIP gold adsorption processes suggests that various factors inhibit the gold adsorption rate and practical loadings in the carbon.
The increased usage of activated carbon in the recovery of precious metals from leach solutions has been well recognized. The specific application of carbon adsorption to more complex gold and silver ores warrants the investigation into the effect of metallic ion interaction with gold adsorption.
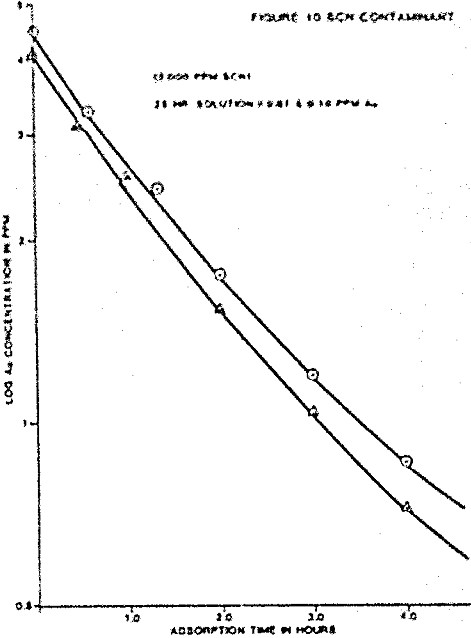
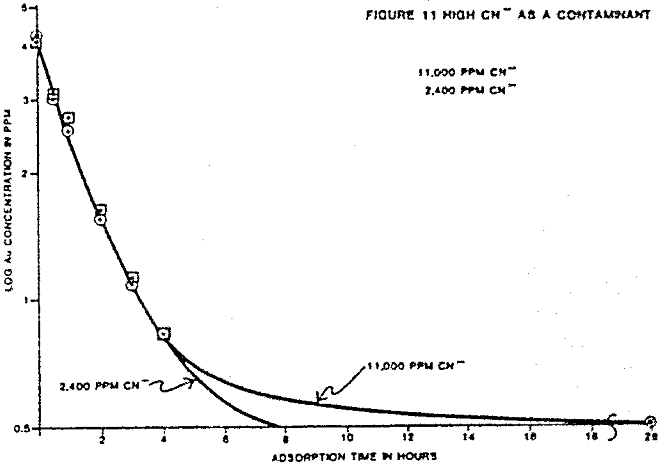
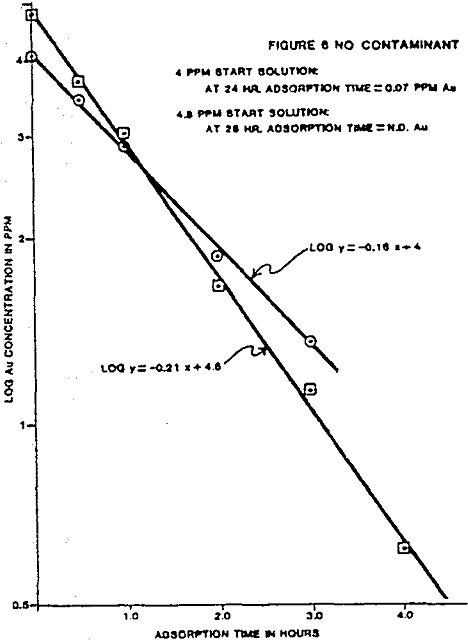
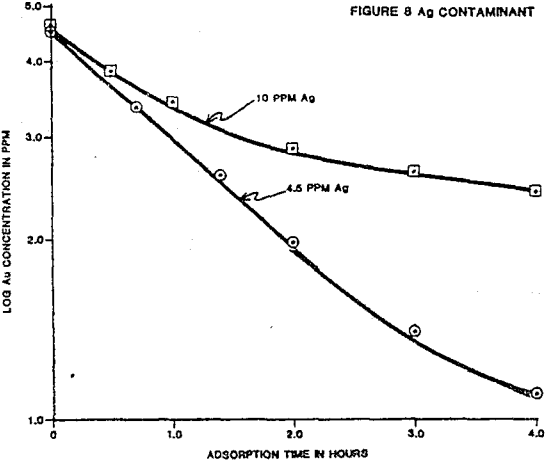
The individual ion effects on gold loading were measured at several different ionic concentrations. First the loading rate from a 4 ppm gold solution in deionized water was determined. The following were held as near constant as possible for each test: solution volume, weight of carbon, temperature, starting solution feed ionic concentration, and agitation rate. All water used to make solutions was deionized before specific ion addition. The measured effects on gold loading for each contaminant are summarized.
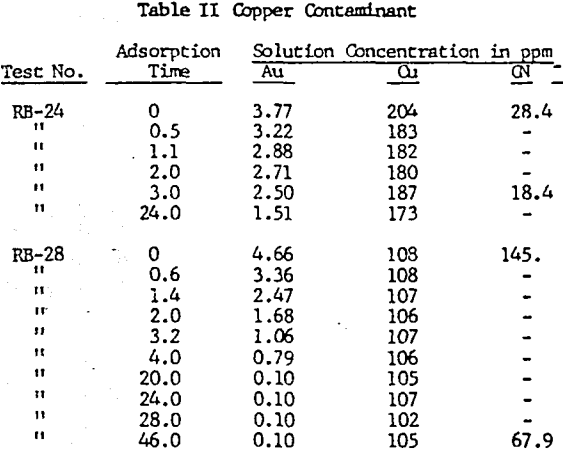
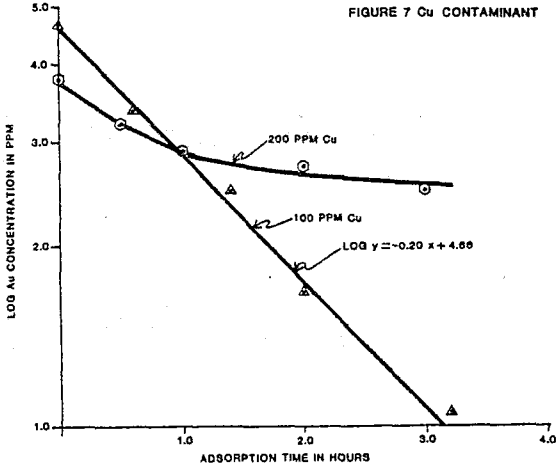
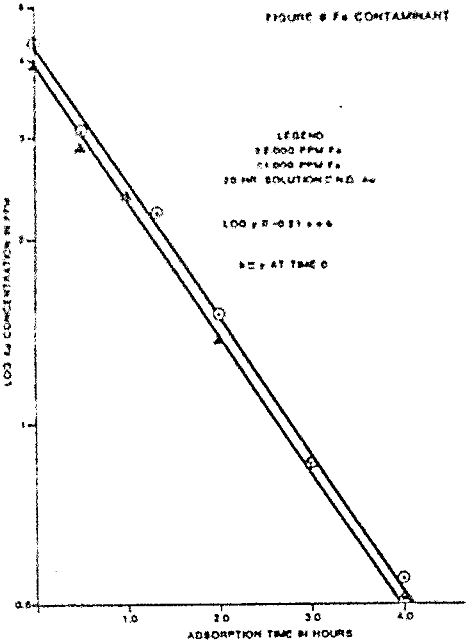
Iron contaminant levels of approximately 2,000 and 1,000 ppm were tested for gold loading rate with a 4 ppm gold solution. The iron contaminant was produced by dissolving sodium ferrocyanide crystals in deionized water.
Ferrocyanide at the two stated levels did not effect the rate of loading. The two tests carbons loaded to 161 and 155 oz/ton gold. The gold metals balance indicated reasonable accountability at 89% and 94% of the starting solution gold contents.