Table of Contents
The U.S. Bureau of Mines developed an alkaline oxidative pretreatment to increase the recovery of gold from refractory sulfide ores containing arsenopyrite (FeAsS) and/or pyrite (FeS2). Pretreatment of a low grade ore containing 0.4% FeAsS, 3.5% FeS2, and 2.74g/mt Au with 1.2M NaOH, 40 psig 02, 100° C, and 4 hr in a 2-L autoclave resulted in 94 to 96% sulfide oxidation. Cyanidation of the pretreated residue resulted in 88% Au extraction compared to <20% Au extraction for untreated ore. Cyanide consumption was 0.25 kg NaCN per metric ton of ore. A flowsheet for treating sulfidic low-grade gold ore is proposed.
During the 1980’s, the gold industry saw strong gold prices precipitating what is termed the “gold rush of the 80s.” The increased activity has resulted in processing of ores previously considered not profitable or those classified as refractory. Refractory ores, such as ores with gold disseminated in pyrite or arsenopyrite, are not amenable to direct cyanidation.
Historically, refractory ores were roasted to oxidize the sulfides. New hydrometallurgical technology developed in the past decade such as aqueous pressure oxidation (Berezowsky, 1984) and bacterial oxidation (Bruynesteyn, 1984) are viable alternatives to roasting.
Oxidation of sulfides from a thermodynamic point of view is feasible over the entire pH range. Since 1985, acidic aqueous pressure oxidation has been used commercially (Berezowsky, 1984,1989). Acidic pressure oxidation of pyritic gold ore requires treatment temperatures between 160° to 210° C and pressures of up to 400 psig. The resulting residue requires thorough washing and neutralization prior to cyanidation. A high degree of sulfide oxidation usually ensures high gold recovery by cyanidation. Current operations which use this technology include McLaughlin Mine, California; Sao Bento Mineracao, Brazil; and Getchell Gold Mine, Nevada (White, 1990).
Alkaline pressure oxidation using NaOH and oxygen, first proposed by Sill (1960), was used commercially for treating a cobalt-nickel arsenic ore (Chilton, 1958). Alkaline pressure oxidation is currently being used at the Barrick Mercur Gold Mine, Utah. The operating conditions at Mercur are 220° c and up to 460 psig pressure and pH between 7 and 9, for treating ore with 1 to 2% sulfur and 2g/mt Au (White, 1990). Recently, electrochemical studies on arsenopyrite oxidation in alkaline media was reported by Hiskey (1987) and Beattie (1987). Taylor (1987) investigated the hydrothermal oxidation of natural arsenopyrite in sodium hydroxide (NaOH) and the effects of several variables on the mineral. The Bureau of Mines (Bhakta, 1989) investigated use of the Sill process for an ore containing 61% arsenopyrite, 9% pyrite, and 54.7 g/mt Au. NaOH was selected based on the results of electrochemical studies (Bhakta, 1989) in which the extent of pyrite oxidation by ammonium hydroxide, ammonium carbonate, and NaOH was evaluated. The alkaline pretreatment, using 2.0M NaOH, 100° c, 40 psig oxygen pressure, and 4 hr reaction time, resulted in 90 to 93% gold extraction compared to 5% for untreated ore. The residue of the alkaline pretreatment is primarily iron oxide and does not require washing or neutralization before cyanidation. The investigation of alkaline pressure pretreatment was continued by the Bureau for ores more typically found in the United states today. This paper presents the results of an investigation to determine the viability of using a Na0H-O2 pretreatment for a sulfidic low grade gold ore from Nevada.
Experimental Procedures
The pretreatment was conducted in a standard 2-L AISI Type 316 stainless steel autoclave with a glass liner and equipped with a 1.5 kw heater and 50 w stirring motor. The head incorporated a stirring assembly, a 100 psig full scale pressure gauge, a thermowell, a solution sampling tube, and a gas inlet and outlet. The stirrer consisted of a 5 cm, six-blade turbine-type impeller positioned at the end of the stirrer shaft and near the bottom of the vessel. A stirring speed of 600 rpm was sufficient to suspend the solids. The solution sampling tube was equipped with a dual-disk stainless steel filter capable of retaining particles larger than 10 µm.
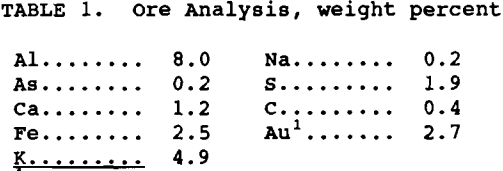
Analysis of the ore, minus 74 µm, is listed in table 1. The ore contained 0.4% arsenopyrite, 3.5% pyrite, and 2.74g/mt Au. All chemicals used were reagent grade and solutions were made with deionized water. Five-hundred milliliters of freshly prepared NaOH solution, pH 13 to 13.5, and ore were added to the reactor. The reactor was sealed, pressurized with oxygen, and heated to operating temperature in 45 to 50 min, with continuous stirring. After each experiment, the slurry was vacuum filtered, and the solution and residue analyzed. Pretreatment parameters investigated were: temperature 80° to 140° C, NaOH concentration 0.6 to 1.2M, pulp density 23 to 38% and reaction time 1 to 6 hr. The oxygen pressure was maintained at 40 psig based on previous results (Bhakta, 1989).
The pretreated residues were repulped to 9% solids and bottle rolled with sodium cyanide for 24 h at room temperature. Initially, 1.5 g NaCN was added per 100 g of ore, but was reduced in later experiments to 0.08 g NaCN per 100 g ore to decrease cyanide consumption. For flowsheet development experiments, the pretreated slurry was cyanided, using 0.08 g NaCN per 100 g ore, for 24 hr.
Results and Discussion
It was determined from previous work (Bhakta, 1989) that temperature and NaOH concentration were the two variables which influenced subsequent gold extraction the most. The effect of temperature at 80°, 100°, and 140° C was tested using 1.0M NaOH, 33% solids, 40 psig oxygen pressure and 4 hr reaction time. The results are given in table 2. Although sulfide oxidation was almost identical for all three temperatures, the gold extraction was lower at 80° C compared to the higher two temperatures. A temperature of 100° C was chosen as the operating temperature for the pretreatment and used for all subsequent testing.
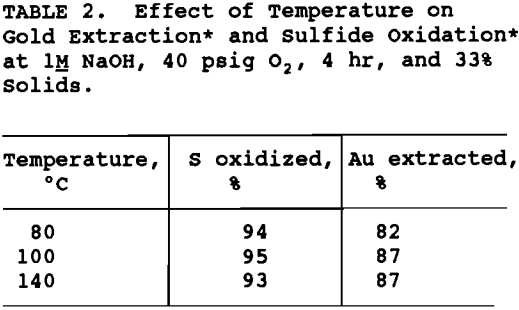
NaOH concentration and percent solids were varied to maintain the ratio of NaOH to ore the same for all tests. As shown in table 3, NaOH concentration was varied from 0.6 to 1.2H and the percent solids from 23 to 38% . The degree of sulfide oxidation and gold extraction remained essentially constant. In order to maximize throughput without decreasing gold extraction significantly, 1.2M NaOH and 38% solids were chosen as operating conditions for the pretreatment flowsheet development.
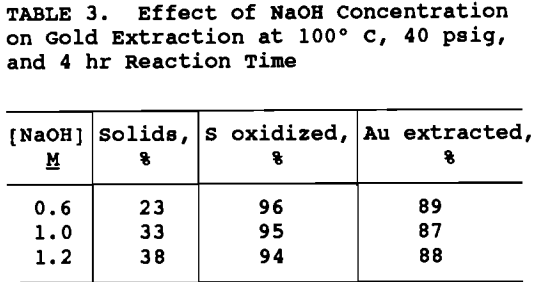
Reaction times between 1 and 6 hr were tested. The results, based on multiple tests, in figure 1 show that maximum gold extraction was achieved when the pretreatment was 4 h. Gold extraction decreased with time thereafter. For a diffusion controlled reaction (Bhakta, 1990), one would expect an increase in reaction time to result in an increase in the oxidation of sulfides, thereby, increasing gold extraction. A possible reason for the decline in gold extraction is the precipitation of gypsum at longer pretreatment times. The ore contains calcium silicates which were found to react with the NaOH resulting in 1 to 4 g/L silicon in solution, but less than 8 ppm calcium, suggesting that the calcium precipitated as gypsum.
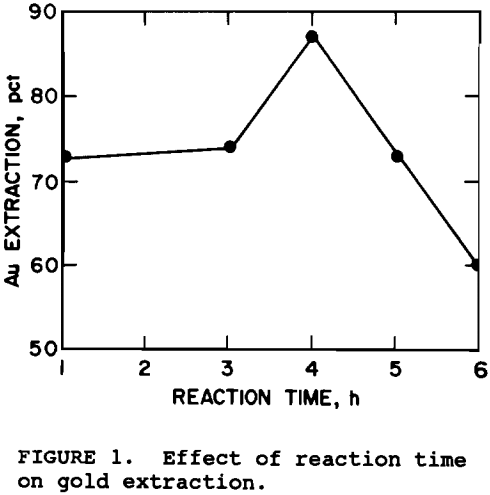
It was concluded from the above data that the best operating conditions for the pretreatment would be 1.2M NaOH, 100° C, 38% solids, 4 hr reaction time, and 40 psig oxygen pressure. Cyanidation of ore pretreated at these conditions resulted in 88% gold extraction with cyanide consumption at 0.25 kg NaCN per metric ton of ore. Cyanidation of untreated ore resulted in less than 20% of the gold extraction.
Proposed Process Flowsheet
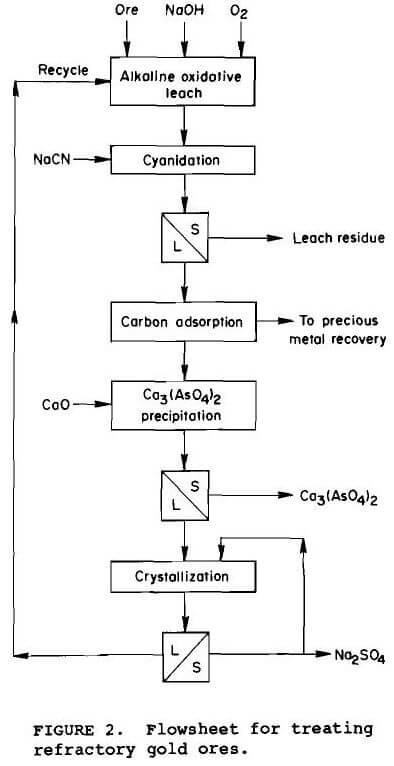
Using the preferred operating conditions defined for alkaline pressure oxidation, cyclic experiments were conducted in which each unit operation was conducted chronologically with the final solution being recycled to the pretreatment leach as shown in figure 2. Since the slurry exiting the alkaline oxidative pretreatment is at pH 13 to 13.5, it is cyanided directly using 0.8 kg NaCN per tonne ore. After cyanidation, the slurry is vacuum filtered. The pregnant solution is passed through a carbon column to recover the precious metal values. To control sodium sulfate buildup, the effluent from the carbon column containing 40 g/L sulfate, 25 g/L sodium, and 1.5 g/L arsenic, is cooled and maintained at 4° C. To initiate crystallization, 32 g of Na2SO4 is added as seed per liter of solution. Approximately 25% of the sodium sulfate in solution is crystallized in an hour. The crystals are filtered from the slurry and the solution is recycled to the alkaline oxidative leach containing 31 g/L sulfate, 18 g/L sodium, and 1.3 g/L arsenic. Precipitation of arsenic with lime before sodium sulfate crystallization controlled the arsenic content of the solution (Bhakta, 1990). The calcium arsenate precipitate passes the 5 mg/L arsenic limit for the Environmental Protection Agency’s Toxicity Characterization Leach Procedure (EPA-TCLP). The sodium hydroxide content of the recycle solution is approximately 2 to 5 g/L and requires makeup NaOH solution for each alkaline pretreatment.
Conclusions
The alkaline pressure oxidation of sulfidic gold ores is advantageous due to its relatively mild operating conditions of 100° C and 40 psig oxygen pressure. This translates to simpler and possibly cheaper materials of construction for the autoclave compared to acidic pressure oxidation systems. The waste streams from this process are environmentally acceptable. Nevertheless, the use of NaOH in the pretreatment needs to be weighed against the mild operating conditions, when evaluating the process as an alternative to treat refractory gold ores.
