The determination of aqueous cyanide is accomplished by either detection in the sample as collected or by removal of the cyanide of the collected sample, followed by detection. The direct determination of the cyanide content of a solution can only be used in the simplest of systems, since many interferences can result in errors. Thus the most widely used method for total cyanide determination involve the reflux distillation of the sample using heat, acid and vacuum or air flow. The basic theory underlying this analytical approach is that by reducing the pH, the quality of molecular hydrogen cyanide will be increased and can be removed from the solution by elevating the temperature. The hydrogen cyanide is then collected in a caustic solution and analyzed.
The general approach of removing the cyanide from the sample by distillation represented a major advance in this area of analytical chemistry. It enabled the determination of cyanide in many additional types of samples. When it became apparent that the tightly bonded cyanide complexes were not being detected by this approach, many methods for complex ion destruction were proposed. These methods usually involved low pH adjustment with oxidizing mineral acids, coupled with the catalytic decomposition of the tightly bound complexes. All of the complexes except the strongest could be broken by these methods. The catalytic reflux distillation step has been found to have some negative aspects, since many materials that can be distilled with hydrogen cyanide will interfere with the color development step and result in a false positive reading.
The EPA method for total cyanide, Storet 720 utilizes a reflux distillation in the presence of a mineral acid and a catalyst. The catalyst is present to aid in the decomposition of tightly bound metal complexes; cuprous ion was used, but magnesium ion has been substituted in an attempt to reduce the thiocyanate ion interference. The procedure for the analysis of total cyanide involves the reflux of an aliquot of sample in sulfuric acid with a metal ion catalysis. This system is refluxed for one hour during which air is bubbled through the solution and then scrubbed in an absorber filled with sodium hydroxide. This scrubber solution is analyzed directly for cyanide. This analysis can be performed by either a titration or a colormetric procedure. The colormetric procedure is used for lower concentrations. The two major areas of difficulty in the procedure are the reflux distillation and the color development. Historically the reason for the distillation is to avoid the physical and/or chemical interference of non-cyanide species in the original collected sample with the colormetric procedure.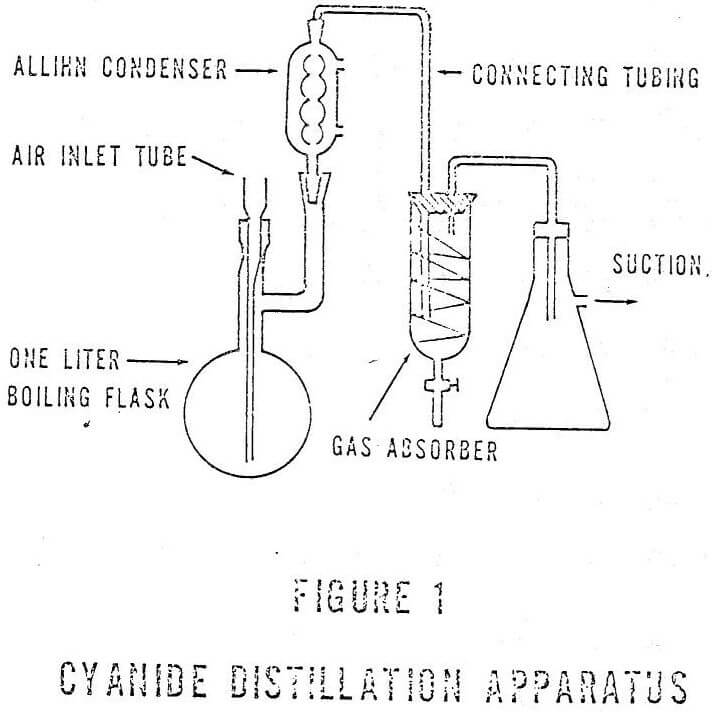
In actual application the reflux distillation is fairly successful in removing the cyanide from the interfering species present in the original sample. However, as efforts have been made to push the method below one part per million and the scope of waste streams being analyzed has been expanded, many unanticipated problems have been uncovered.
These problems affect both the precision and accuracy of this procedure. Because of the large number of manipulations.
That are required to carry out this analysis, it is necessary to give careful attention to analytical techniques, especially when the method is being used for samples below 1 ppm. The range of factors introducing errors in the precision of this method goes from the manipulative aspects such as pipetting to the introduction of contamination from external sources. All of these problems are encountered even when working with laboratory generated standard solutions.
When real world samples of industrial effluents are analyzed the problems associated with the precision of the method are greatly magnified by considerations affecting the accuracy. The accuracy of this method is heavily influenced by interferences such as thiocyanate ion and trace metals. Reducing agents also can present considerable problems.
The major interference appears to be the thiocyanate concentrations present in ore dressing waste streams. In general, the water from an ore dressing process is very high in its thiocyanate ion content, having concentrations in the range of 10 to 200 ppm. This is not unexpected, since the combination of cyanide ions and the finely divided metal sulfides in a basic solution is expected to result in the production of thiocyanate ions. The high pH of most ore dressing processes and the fine small particle size makes the formation of the thiocyanate ion both thermodynamically and kinetically favored.
The addition of strong solutions of sulfuric acid to thiocyanate salts results in the formation of hydrogen sulfide which will also distill from the reflux vessel and be collected in the caustic scrubber. While it is uncertain as to the exact precise nature of the sulfur specie leaving the reflux flask, there is no question of its presence and that it produces an interference in the color development step that is impossible to quantify.
One of the reasons for the replacement of the cuprous ion with magnesium ion as the catalyst was to suppress the interference resulting from the thiocyanate decomposition. However, ore dressing waste streams contain low concentrations of the cuprous ion making the catalyst change of questionable value. This interference is an extremely serious one since thiocyanate solutions have shown as high as ten percent conversion. Thus, in situations when thiocyanate concentrations are in the range of 200 ppm only one one hundreth of a percent has to be in the capacity of an interference when the method is being used to evaluate compliance with a 0.02 ppm cyanide limitation. It is possible to generate laboratory samples containing no cyanide that when subjected to this analytical procedure will result in apparent significant violations of the 0.02 ppm level.
Efforts to prevent the thiocyanate interference in the reflux distillation have been ineffective. This results from the uncertain nature of the reaction products generated in the reflux flask, one of which is hydrogen cyanide. Thus, solutions containing less than one ppm of cyanide cannot be accurately analyzed by the reflux method, because the “cyanide” produced by the thiocyanate decomposition cannot be differentiated from that in the sample and may be present in greater quantity.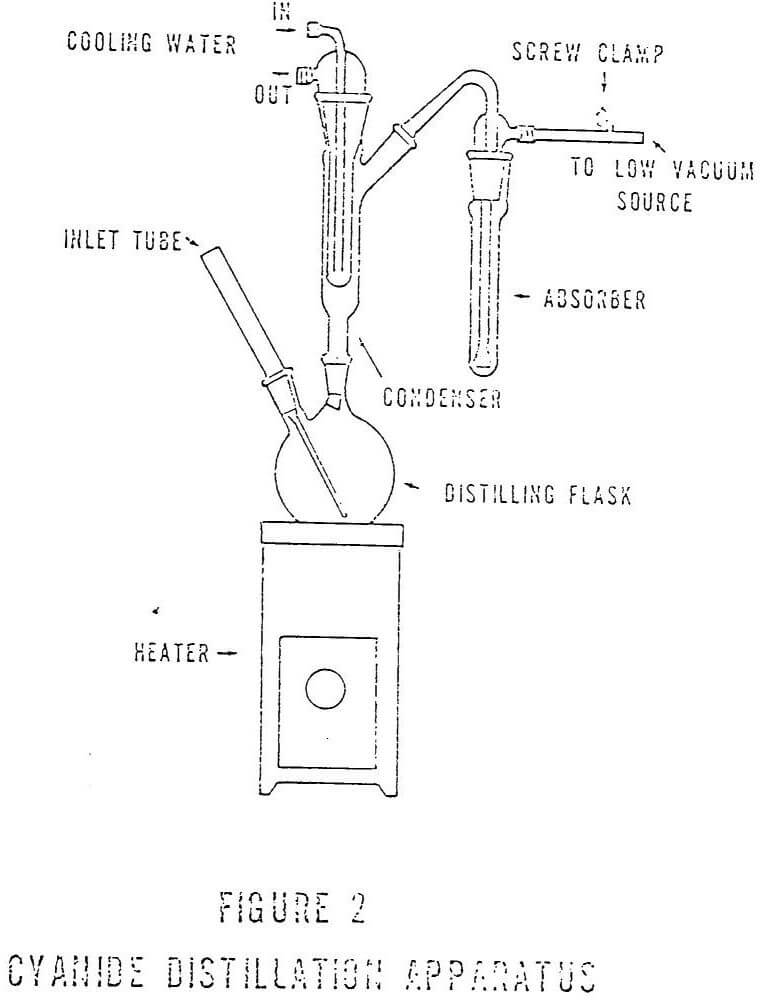
A round robin study conducted by the EPA and Radian Corporation with the help of several industrial labs tried a lead acetate scrubber after the distillation flask and before the sodium hydroxide absorber to prevent sulfur species from entering the caustic absorption solution and consuming the chloramine-T. If thiocyanate is still intact after the distillation, this might prevent the false readings. This does not appear to be the case and the interference persists. The findings of the round robin study indicated that the lead acetate scrubber method was as good as the EPA standard method.
Cadmium nitrate has been used to precipitate the sulfur as cadmium sulfide. This procedure involved the addition of the cadmium directly to the absorber, with subsequent filtration before the color development. The cadmium sulfide collected not only plugged the fritted cylinders, but also required filtration of the solution and loss of cyanide by adsorption on the precipitate. It also adds steps to an already involved procedure.
The distillation flask represents a system in which strongly bound complexes are dissociated, with the aid of catalysis, under very acid and oxidizing conditions. This set of conditions can cause undesirable chemical reactions to occur; especially in the presence of thiocyanate and metal sulfides which can cause the evolution of hydrogen sulfide, hydrocyanic acid and hydrothiocyanic acid. The hydrogen sulfide and hydrocyanic acid result from the decomposition of the thiocyanate ion in this strong oxidising system, while the hydrothiocyanic acid is evolved directly from the system. These interferences have been recognized widely and can be eliminated by some modifications to the method.
Efforts to eliminate the thiocyanate interference have been successful when the acidity and oxidizing character of the solution in the reflux container have been reduced. These reductions, which are often accompanied by the removal of the catalyst, have the effect of preventing the decomposition of the thiocyanate ion and preventing the dissociation of the tightly bound metal cyanide complexes. These methods can not generally be used to determine the “total cyanide” content.
In addition to the thiocyanate interference the presence of naturally occuring solids in ore mining and dressing waste streams also causes a problem. This should not be a surprise to anyone since the presence of sulfides during the color development has long been recognized as a problem. There sulfide ion may react in a comparable manner to the cyanide, resulting in a positive interference. In fact, the EPA procedure discusses the necessity of the removal of any soluble sulfide from the sample. It also states that any precipitated sulfides must be removed by filtration prior to the reflux distillation. The reason, for the complete removal is to prevent the sulfide from being dissolved in the hot acid solution and forming H2S. Since hydrogen sulfide has only limited solubility in water, it would be a part of the gas stream leaving the reflux flask and be collected in the scrubber. If it is necessary to remove the precipitated sulfide prior to reflux, it must also be necessary to remove all of the insoluble sulfides that were present in the original sample. Since ore dressing waste streams come in contact with finely divided metal sulfides, and contain these in suspension, it is essential that these samples be filtered prior to reflux. Thus, it is not possible to make a determination of total cyanide for these types of samples. The manner in which these samples are treated, unfortunately, varies from laboratory to laboratory. For example, some laboratories routinely filter any sample from an ore dressing facility in order to avoid this interference, while others are unaware of the problem. The lack of general agreement among results from various laboratories is quite understandable.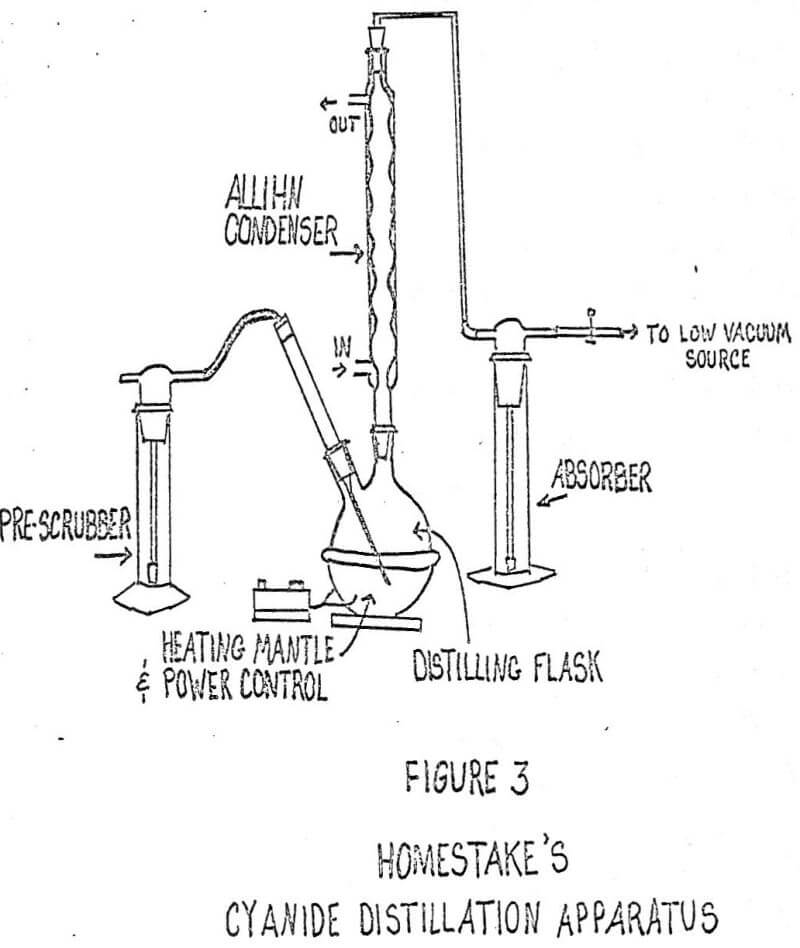
A review of the data generated by several analysts working 0.20 ppm standard cyanide solutions over a period of one year, revealed a mean ranging from 0.248 to 0.136. This was accompanied by a standard deviation ranging from 0.087 to 0.44. These data were ranked and plotted on normal probability paper. It was interesting to note that while all analysts were demonstrating poor precision the probability plots indicated that these poor results were the result of at least two outside and intermittent factors. As a result of this review indicating that the lab was not able to obtain acceptable precision with this method on laboratory generated samples having a concentration of 0.20 ppm cyanide, an extensive effort was made to uncover all possible sources for this difficulty.
The analysis for total cyanide includes a distillation step with absorption in NaOH and subsequent detection of cyanide by titration or colormetric procedures. Suggested aparati for cyanide determinations are shown in Figures I and II. A combination of these two configurations appears to be more useful in the analyses than either of the other two. Figure III shows this configuration.
Gold processing includes the cyanidation of ore. For this reason, cyanide is prevalent in the atmosphere near gold processing plants and produces an analytical handicap which can be dealt with fairly well with the use of sodium hydroxide pre-scrubbers. If some contamination escapes the pre-scrubbers, a graphic presentation of results will show if there was the slightest bit of contamination that might otherwise have been overlooked. Various sources of contamination and interference can be pinpointed this way.
A prescrubber has been incorporated into the set-up to prevent contamination from airborne cyanide. A 1.0 normal sodium hydroxide scrubbing solution appears to be sufficiently strong to prevent the largest part of the contamination from entering the boiling flask. Detection of cyanide contamination and effects from other sources will be discussed later in this paper.
Wherever possible, ground glass joints should be used to prevent leaks caused by hardening of rubber stoppers. The slightest leak will disrupt the airflow, prevent proper absorption, and allow for external contamination.
The vacuum on the system used for total cyanide makes heating of the boiling flasks difficult. A bunsen burner or similar open flame device will provide very hot and very uneven heating quickly. Quick heating will produce great pressure and cause one of three results:
(1) The air inlet tube will break off, (2) the pressure will force the flask contents up the air inlet tube, or (3) the flask will break, sometimes violently. Electric heating mantles with power controls have proven to reduce the probability of these unwanted occurrences. The heat produced is not as sudden as the flame and is much more even, reducing the likelihood of an exploding or cracking flask.
The Fisher Milligan scrubber shown in Figure I is less efficient than the other types of absorbers and should be replaced with scrubbers having fritted cylinders or discs and ground glass joints. Apart from being much easier to handle, these fritted scrubbers are not subject to the constant chipping common with the Fisher-Milligan scrubbers and are much more efficient. Repair of the chips with non-cyanide containing substances was a major concern.
Maintenance of the steady flow of air through the scrubbers is still an important consideration; however, the use of fritted cylinders renders the necessity for an extremely constant flow less critical. The bubbles formed by the frit provide more contact surface than the larger bubbles from the Fisher-Milligan scrubbers and are much easier to control.
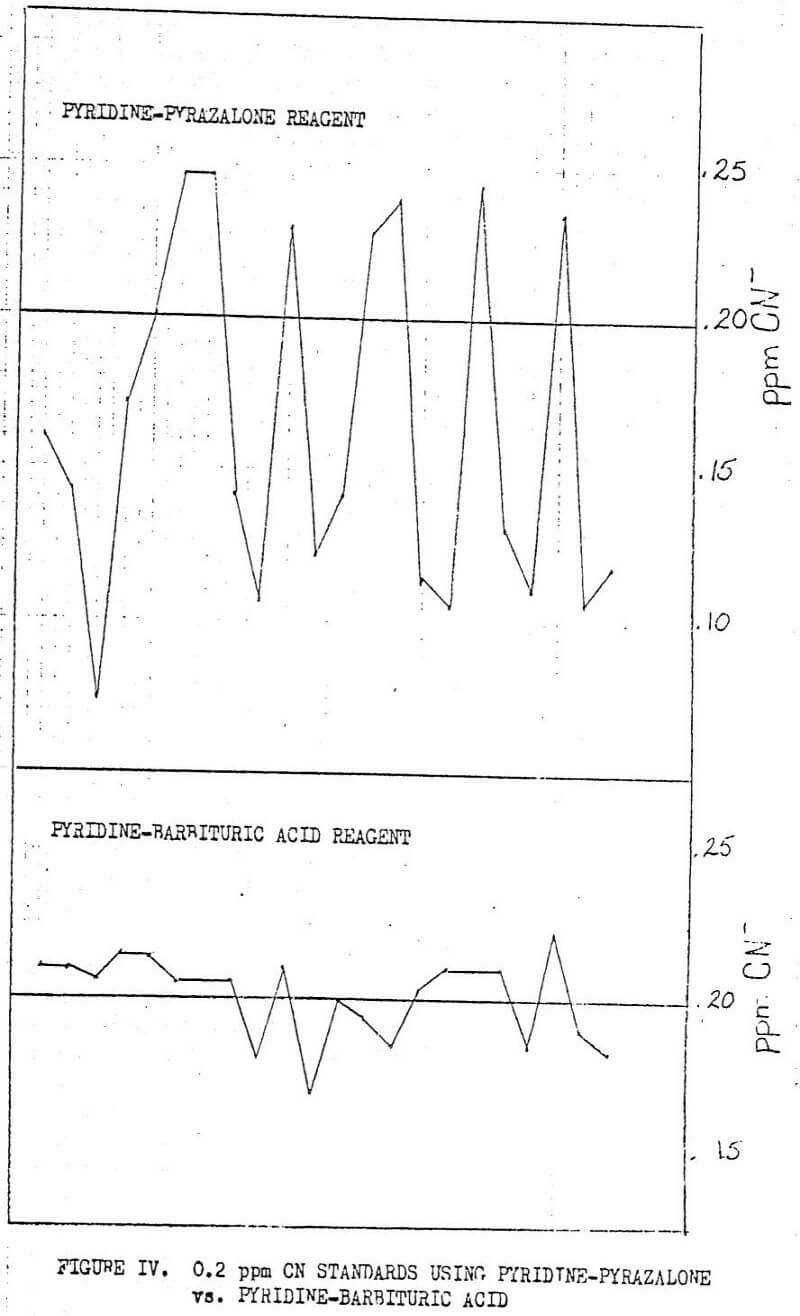
The method for total cyanide determinations widely used before the present method include the use of pyridine-pyrazalone in the colormetric step. The elimination of the use of the pyridine-pyrazalone reagent from the color development stage of the total cyanide method improves the reliability of the method tremendously. Figure IV shows the immediate improvement in precision and accuracy with the change to pyridine-barbituric acid in the coloring step. With the change in the pyridine reagent also came the increase in the amount of chloramine-T from 0.20 ml to 2.0 ml to minimize the effects of reducing agents in the absorption solution. There are no reducing agents present in the laboratory generated solutions seen in Figure IV, so that the improvements in precision and accuracy can be interpreted as a direct result of the change in the pyridine reagent.
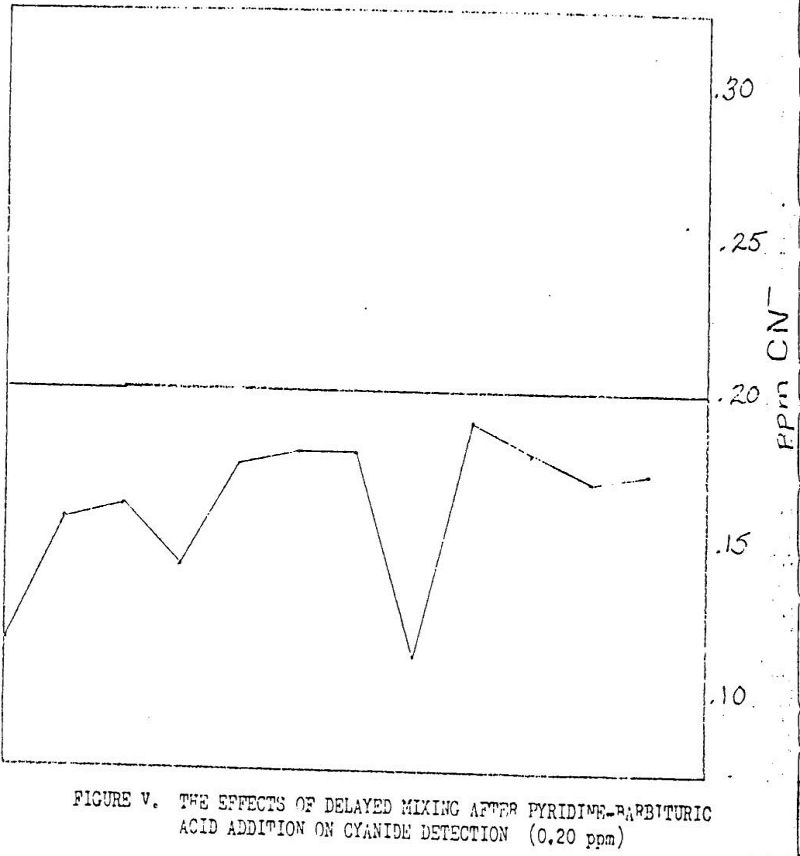
The probability of producing accurate, and most importantly, reproducible results using plyridine-pyrazalone was highly unlikely. This was due to several factors, one of which was the timing of the addition of reagents in the coloring step. For example, if the samples are colored in an assembly line fashion, the time delay between the mixing of the first sample after reagent additions and the mixing of the last sample could cause as much as a 50% error, see Figure V. Timing with the new reagents is still important, but the effects are much less obvious.
Any lab using cyanide or analyzing for cyanide has the potential for contamination of samples. Cyanide will begin to volatilize at pH 10.5 and continue rapidly with a decrease in pH. Therefore, air-borne contamination is a very real possibility. If leaks are allowed to form in the refluxing system, some of this contamination will be apparent in the results of the determinations performed during that time. Again, ground glass joints are encouraged, as rubber stoppers tend to harden and produce air leaks.
With each set of samples run, the analyst runs a blank and a cyanide standard. The analyst then makes up a reagent blank and a cyanide standard of the same concentration as that being refluxed. That will make two standards and two blanks. When the coloring of all samples, blanks and standards is completed, four comparisons are made:
- the refluxed standards vs. refluxed blank.
- the refluxed standard vs. unrefluxed blank.
- the unrefluxed standard vs. unrefluxed blank.
- the unrefluxed standard vs. refluxed blank.
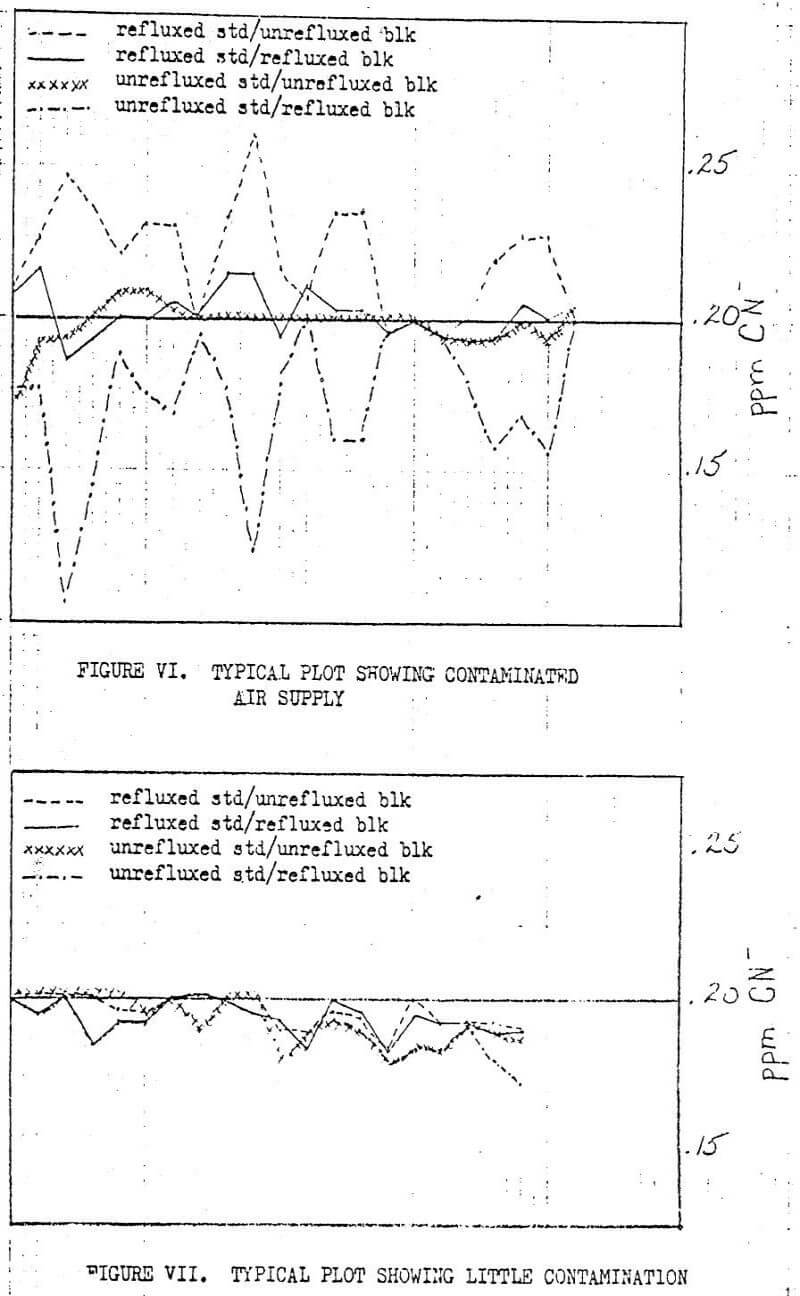
When these four comparisons are plotted on the same set of axes, a pattern will usually develop which indicates if there was an interference. Figures VI and VII show sample plots of these four comparisons as Homestake uses them to monitor its operators as well as the atmospheric contamination as it affects results.
Many factors enter into the analysis of these plots. Included in the list of possible points of error are:
– contaminated or deteriorated reagents or standards
– contaminated air
– contaminated glassware
– operator errors (pipetting, measuring, etc.)
– equipment failure
Logical evaluation of the plotted points will eliminate the possibilities which are not applicable and narrow the points of error down to one or two.
Accuracy and precision can be followed precisely with the plots of the four comparisons above. The effects of new apparatus, new operators, poor reagents and contamination are easily spotted and corrected, which help improve results. However, accuracy and precision can be no better than the method itself. At present there does not appear to be a reliable method available for the determination of total cyanide in ore dressing waste streams because of the problems affecting the precision in addition to the interference resulting from the presence of thiocyanate and sulfides.
It is encouraging to be able to report that the efforts that we have discussed have resulted in a significant improvement in the precision of the results obtained from 0.20 ppm standard solutions. In the past few months we find that based on 246 analyses the mean is 0.1955 showing a standard deviation of 0.0094. This gives a 99% confidence level for 0.20 standard solutions of 0.1955 plus or minus 0.0015. This type of precision has resulted only from a concerted effort to carefully watch all aspects of analytical technique involved in this complicated procedure, in addition to use of items such as prescrubbers to minimize contamination from external sources. It should, however, be pointed out that this positive improvement in precision does not address the difficulties previously discussed that had impact on the accuracy of the method. This remains an unanswered question and one of major significance to the ore dressing industry.