Table of Contents
Etching solutions containing hexavalent chromium ions (e.g., chromic acid) are used in brass finishing, printed circuit board etching, preparation of plastic for plating, anodizing, and various other industrial surface treatment operations. As the solutions are used, hexavalent chromium ions are reduced to their trivalent state, contaminants accumulate in the solution, and the effective acid concentration is reduced. When the etching rate becomes unsatisfactory for the particular operation, the solutions are discarded. This is a problem because the discarded solutions are pollutants, and because replacing the etching solution is expensive. The Bureau of Mines has developed a process for regenerating these spent solutions and returning them to the surface treatment operation without interrupting the etching process.
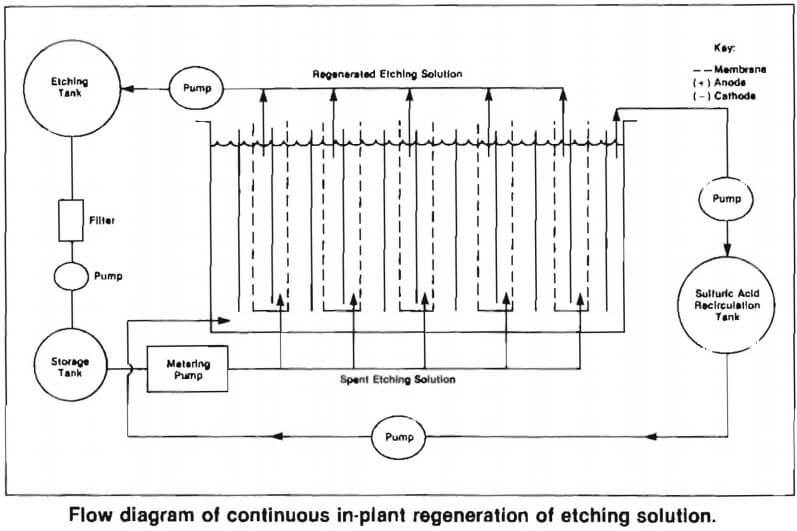
An electrolysis cell containing anode and cathode compartments that are separated by permeable membranes, is used for regenerating the spent solutions. The spent chromic acid solution is fed to the anode compartment and sulfuric acid is circulated in the cathode compartment. Application of a steady electrical potential of a few volts dc across the electrodes causes the copper ions and other contaminants in the spent solution to migrate through the membrane to the cathode compartment where copper is plated onto the cathode. Zinc and the other contaminants remain in the sulfuric acid. At the same time, the complex, trivalent chromium ions in the spent solution are attracted to the anode where they are oxidized to their original hexavalent state. The regenerated solution in the anode compartment is then recycled back to the plant’s etching operation for reuse.
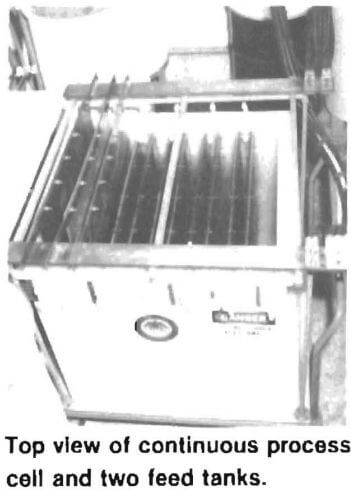
Installation of a continuous industrial-sized regeneration process is simple, and little space is required. In the research unit shown in the photograph, the cube-shaped tank is the electrolytic cell. It was designed to continuously regenerate solutions for an industrial brass etching tank of 1,000 gallon capacity. In addition to the electrolysis cell, the installation includes a source of direct current and two cylindrical tanks. One tank is used for holding sulfuric acid solution, small amounts of which are required as a makeup feed to the solution surrounding the cathode. The other tank is used as a surge tank for the feed of waste chromium solution.
Plumbing requirements are minimal. Piping from the chromium etching tank to the surge tank is required; a return line for the regenerated chromium completes the solution circuit. See flow diagram.
Test Results
The chromium regeneration process was successfully applied to various chromium bearing solutions on a laboratory scale at the Rolla Research Center for more than two years. Next, the regeneration unit shown in the photograph was designed and constructed to specifications developed from these laboratory tests. That unit operated successfully during 5 weeks of rigorous shake-down tests in which industrial waste chromium solutions from two companies were regenerated for recycling. From 20 to 40 percent of the metal impurities in those waste solutions were removed, depending on the flow rate of the solution through the cell. The regenerated solutions were as effective as fresh solutions for etching brass, and were superior for removing red stain from brass parts.
Consumption of electric power was modest. Higher solution flow rates lowered the power consumption per unit of regenerated chromium, but also reduced impurity removal.
To demonstrate the feasibility of the process, the regeneration unit was installed and operated for a 2-week period at each of two industrial plants during 1980. At each plant, the normal practice was to discard the spent solutions daily. Operation of the unit demonstrated that regeneration and reuse of spent solution was practical and that chromic acid consumption and disposal could be significantly reduced.
This research was done by personnel of the Bureau of Mines Research Center at Rolla, Mo. The Project Officers are Mr. Lawrence George and Mr. Andrew Cochran.
Patent Status
United States Patent Application Serial No. 37,089 covering the design of the electrolytic cell was filed on May 8, 1979. For further information write to the Department of the Interior, Office of Solicitor, 18th & C Streets, N.W., Washington, D.C. 20240.
For More Information
A Bureau of Mines Report of Investigations 8377, “Regeneration and Recycling of Waste Chromium Acid- Sulfuric Acid Etchants,” is available. Other information on the subject is being assembled for publication. For further information, or to ask specific questions, telephone 314/364-3169 or write to:
Technology Transfer Officer Rolla Research Center Bureau of Mines P.O. Box 280 Rolla, MO. 65401