Table of Contents
Dielectrophoresis, or dielectric separation, can be defined as the separation of particles according to their dielectric properties. Directly related to the dielectric properties of a particle is the particle’s dielectric constant (K). The difference in K of minerals is often much greater than the differences in density, magnetic susceptibility, or electrical conductivity. To date, several investigators have demonstrated that a mineral placed in a liquid of lower K can be attracted to one electrode in a high-gradient electric field. A mineral with a lower K than the liquid is repelled. This technique could, therefore, provide a method of mineral separation not readily attainable by more conventional means.
The first application of such a dielectric separation technique was developed by H. S. Hatfield to separate cassiterite from a mostly quartz gangue. A pilot plant was built as a result of this work, but the project was abandoned when the price of tin fell too low to justify the process. Since that time a number of different kinds of apparatus for dielectric separations have been developed for batch and continuous separations. Generally the batch apparatus is small, quite simple, and used for analytical purposes. Several small-scale continuous dielectric separators have been developed, but most of these separators have design limitations that prevent scaleup. To advance minerals technology, therefore, the Tuscaloosa Research Center of the Bureau of Mines, U.S. Department of the Interior, has designed, constructed, and patented a novel continuous dielectric separator. This report presents the findings of laboratory test work with this separator in order to evaluate the separator’s performance and to establish a number of operational parameters.
Under its program of advancing minerals technology, the Federal Bureau of Mines has invented a laboratory apparatus for continuous separation of minerals based on differences in dielectric properties. The separator consists of a rotating drum electrode and a wire screen electrode positioned 3 mm apart. A high voltage applied across the electrodes that are immersed in a dielectric fluid produces a high-gradient electric field. The high-dielectric-constant (K) minerals are attracted to and conveyed through the separator by the rotating drum electrode, while the low K minerals settle through the screen electrode. Several design and operating parameters, such as electrode configurations, the dielectric liquid and its dielectric constant, electrical voltage and frequencies, feed rates, and particle sizes were studied. In addition, 28 different mineral mixtures were tested with the dielectric separator. In typical tests, a sample of rutile and quartz gangue containing 10-percent rutile was concentrated to 62-percent rutile with 94-percent rutile recovery. A second-stage dielectric separation of the rutile concentrate produced a 92-percent-rutile concentrate. The overall rutile recovery for the two-stage dielectric separation was 86 percent.
Dielectric Separator Design
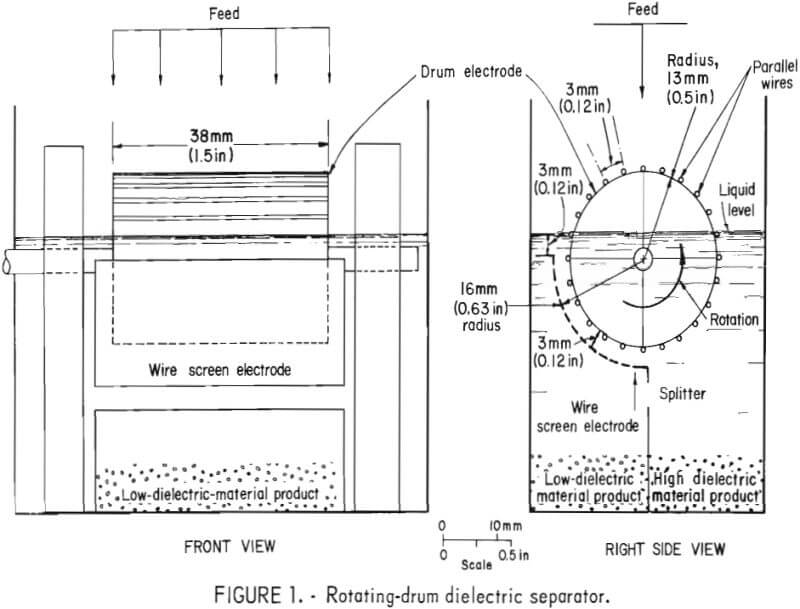
The major novelty of the Bureau’s separator, called the “Rotating Drum Dielectric Separator,” is the high-voltage electrode configuration shown in figure 1. The drum electrode consists of a series of parallel wires mounted on the surface of a 25-mm-diameter insulated cylinder. The wires are spaced 3 mm. apart and parallel to the cylinder’s axis. The screen electrode is a wire mesh shaped into a quarter cylinder having a radius 3 mm larger than the radius of the drum electrode. The screen electrode is positioned as shown in figure 1 with the same center of curvature as the drum electrode.
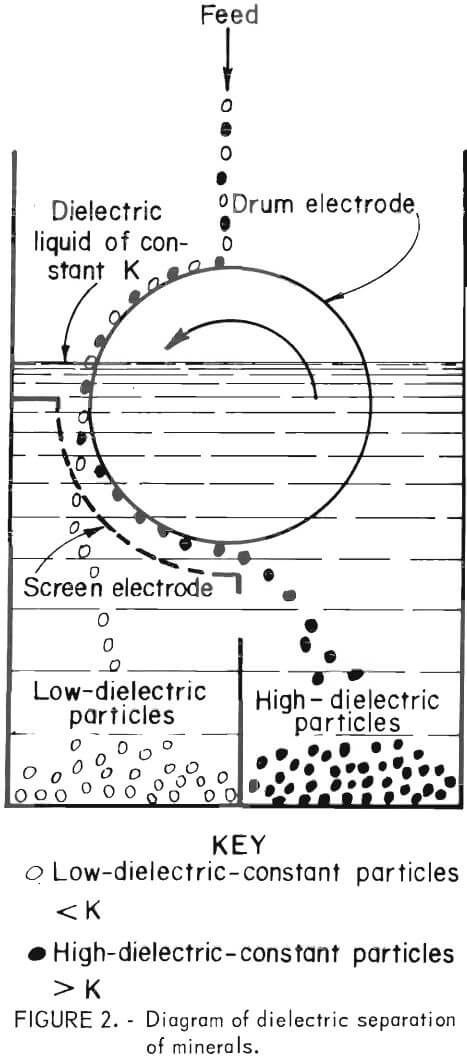
Rotating Drum Dielectric Separator Operation
The separator is designed so that the drum electrode is placed in a dielectric fluid until the fluid level is about 3 mm above the drum’s axis.
The screen electrode, on the other hand, is completely immersed in the dielectric medium. A high-voltage alternating current, applied to both electrodes, produces a high-gradient electric field in the 3-mm gap. The drum revolves mechanically toward the screen electrode. Finely divided mineral particles fed to the top of the drum are carried by the drum’s rotation into the dielectric medium and between the two electrodes. See figure 2 for a schematic diagram of the operational design. Mineral particles having a higher K than the dielectric fluid are attracted to the drum electrode and are carried on the drum’s surface until they leave the high-gradient field. The particles having a lower K than the fluid fall through the screen electrode. In this
manner, higher K particles are continuously moved through the separator and collected beyond the screen electrode, while the lower K particles are collected beneath the screen electrode.
Design and Operating Parameters
Several design and operating parameters were studied, including electrode configuration, dielectric liquid, dielectric constant, electrical frequency, feed rate, and particle size.
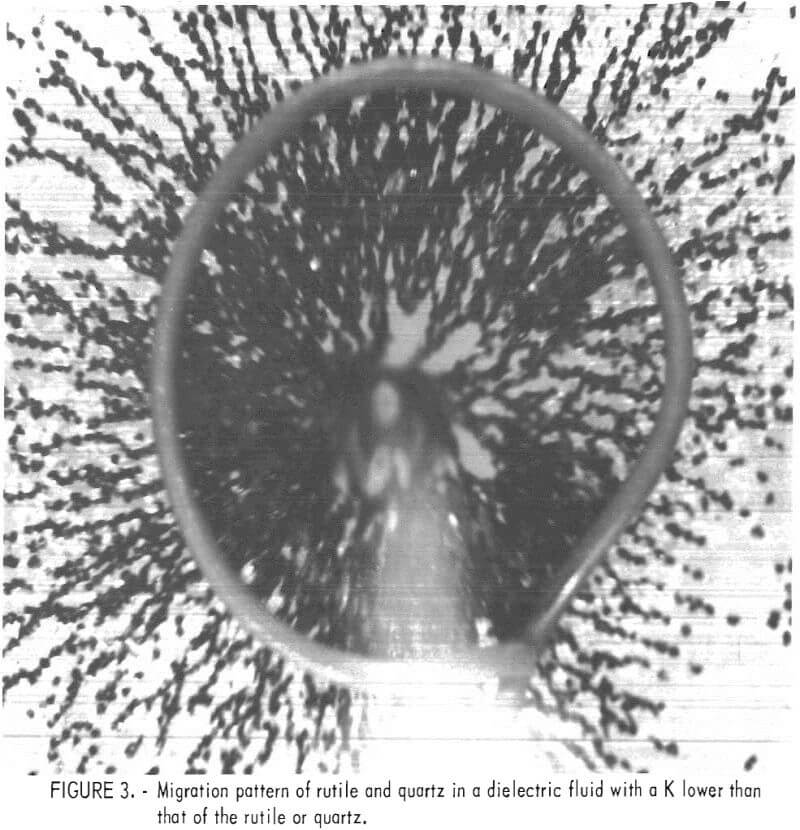
The rotating drum dielectric separator was initially evaluated with a rutile and quartz mixture containing 10 percent rutile. Both minerals were completely liberated. The K of the rutile and of the quartz was measured by observing those particles placed between a 10-mm-diameter wire ring electrode and a wire point electrode centered within the ring. The particles were immersed in pure carbon tetrachloride with a K of 2.24. About 750 volts at 450 hertz was applied to the two electrodes. All of the particles migrated to the point electrode, indicating that their K was greater than the K of the pure carbon tetrachloride. Figure 3 shows the migration pattern for a mixture of rutile and quartz. Ethanol with a K of 26.1 was titrated into a known volume of carbon tetrachloride until particle migration ceased. All such experiments, by the way, were conducted in a microscopic field to accurately determine mineral movement. At the titration end point, the K of the particle equaled the K of the carbon tetrachloride-ethanol mixture. The K of the mixture was calculated from the volume-percent of each liquid and from the K of the pure liquid components. Several replications of these experiments established dielectric constants of 8.0 for rutile and 4.0 for quartz.
The feed was screened into four sized fractions: minus 65 plus 100 mesh, minus 100 plus 150 mesh, minus 150 plus 200 mesh, and minus 200 plus 400 mesh.
Each fraction contained roughly 10 percent rutile, ranging from 9 to 11 percent. Particles larger than 65 mesh tended to bridge the openings of the screen electrode and to block the flow of the low K particles through the screen electrode. Minus 400-mesh particles remained in suspension with the dielectric fluid and could not be separated effectively.
The rutile-quartz mixture was used to study the separator’s operating parameters, such as variations of the drum and screen electrodes, dielectric fluids and the K of the fluids, the voltage and electrical frequency applied to the separator, the drum rotation speed, and feed rate.
Test Procedure
Each of the tests followed the same basic procedure:
- The dielectric fluid at the selected K was placed in the separator, and the voltage and electrical frequency were applied to the circuit.
- The rotational speed of the drum electrode was set.
- The vibrating feeder used to distribute the mineral mixture uniformly upon the drum electrode surface was set.
- Glass trays were positioned below the beyond the screen electrode to collect the separated products.
- The separator was operated about 5 minutes or until sufficient products for analysis and test reproducibility were obtained.
Figure 4 shows the overall apparatus, while figure 5 shows a closeup of the separator during a typical test on the dielectric separation of rutile from quartz.
After each test the products were washed into a filter and rinsed to remove the dielectric fluid. After drying, the products were analyzed by heavy liquid methods for rutile. To determine the experimental accuracy of the system and to demonstrate reproducibility, several of experimental tests were repeated. Operating conditions in the exploratory studies were as follows:
1,000 volts at 450 hertz; dielectric fluid with K of 4.5; drum electrode speed of 8 rpm; mineral feed rate at about 1.0 to 1.5 grams per minute; and a convenient particle size of 100 by 150 mesh.
Basic Design Parameters
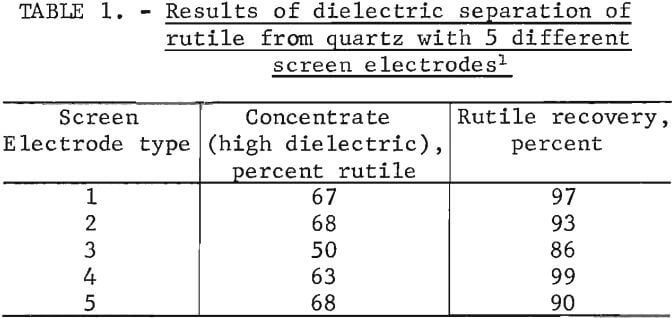
The basic design of the Bureau-developed separator included a wire grid on the surface of the drum electrode and a curved screen electrode positioned 3 mm from the drum electrode. Several types of screen electrodes were evaluated. A schematic and description of each screen electrode are shown in figure 6. The results of the dielectric separations are shown in table 1. All five screen electrode types concentrated the rutile with good recovery. The experimental reproducibility of the dielectric separations was within plus or minus 7 percentage points for the rutile concentrate grade and within plus or minus 3 percentage points for the rutile recovery for 95-percent confidence interval. Screen electrode types I, II, and IV recovered the most rutile and also produced a high grade rutile concentrate. Considering the experimental reproducibility either type I, II, or IV could produce good results.
During the tests with electrode types I, III, IV, and V, the low K particles were trapped on the screen electrode. Eventually enough material accumulated so that the drum electrode, began to rake the material through the separator. To overcome this buildup the separator was periodically vibrated to release any trapped particles. Since the type II electrode did not have a low K particle buildup, it was selected for the subsequent research.
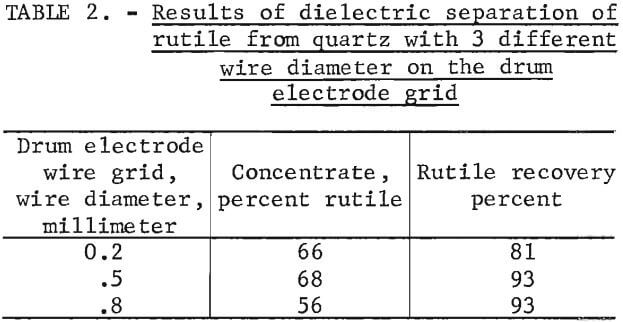
The wire diameter of the drum electrode was varied between 0.2 mm and 0.8 mm with the type II screen electrode. The results of these tests are shown in table 2. The best rutile grade and recovery was produced with the 0.5-mm-diameter wire for the drum electrode. Most of the subsequent experimental work was, accordingly, conducted with the 0.5 mm diameter wire for the drum electrode and the type II screen electrode.
Several dielectric fluids with a K of 4.5 were tested with the dielectric separator. The rotation speed of the drum electrode was 8 rpm. And 1,000 volts at 450 hertz was applied to circuit. The average feed rate to the separator was about 1.5 grams per minute. Nitrobenzene and kerosene, the best mixture of dielectric liquids used in these tests, produced a 68-percent- rutile concentrate with 93-percent-recovery. The results are shown in
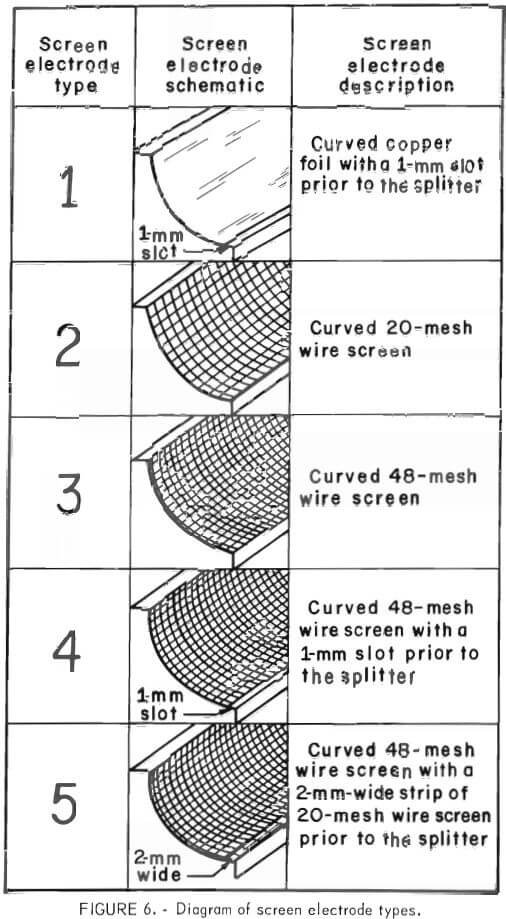
table 3. Again the experimental reproducibility for the rutile concentrate grade was within plus or minus 7 percentage points and between plus or minus 3 percentage points for the rutile recovery, for a 95-percent confidence interval. Several liquid mixtures such as ethanol-xylene and propanol-xylene produced results similar to the nitrobenzene-kerosene mixture within the same experimental reproducibility. The highly toxic nature of many of these dielectric liquids favors the use of the ethanol or propanol mixtures for commercial dielectric separations.
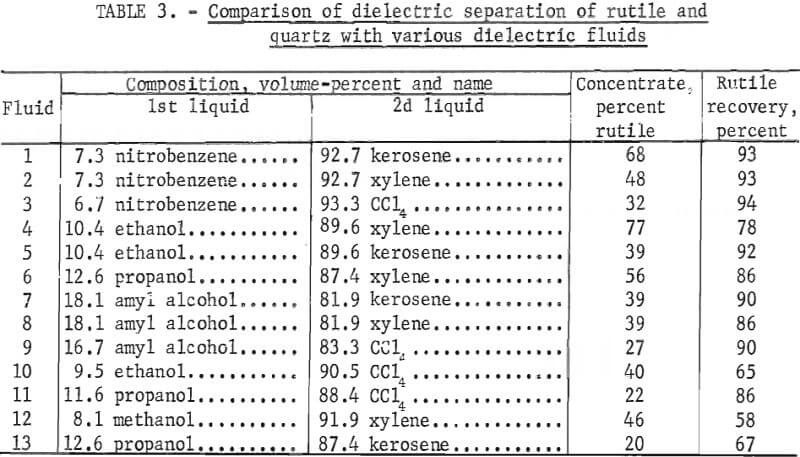
Dielectric separation of the rutile and quartz mixture should be possible using a medium with a K anywhere between 4.0, the K of quartz, and 8.0, the K of rutile. Figure 7 shows the average response in the terms of rutile grade and recovery as the medium K was varied from 4 to 8. The system produced the highest rutile grade at a K of 6.0. The rutile recovery gradually declined as K increased. Above a K of 7.0, rutile recovery dropped sharply. The 4.5 K medium gave the best rutile recovery. Since the rutile grade was at least 4 times larger than the feed, rutile recovery was the basis for selection of a 4.5 K as the best K for dielectric separation of rutile from quartz.
In all of these tests, 1,000 volts at 450 hertz was applied to the circuit. Tests at other voltages were also conducted; however, in the 1,000 to 3,000 volt range, the voltage applied to the circuit appeared to have little effect on the dielectric separation. Below 1,000 volts rutile recovery dropped sharply. Above 3,000 volts turbulence developed between the electrodes also decreasing rutile recovery. Similar responses were noticed also at other frequencies as well.
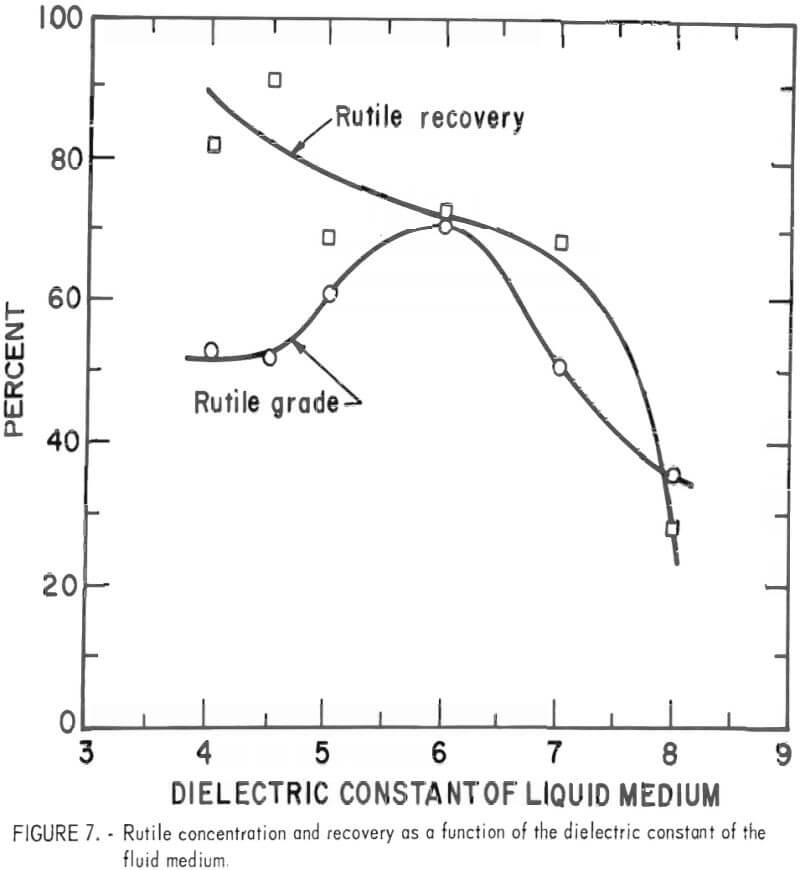
The separation response to electrical frequencies was also tested. At 1,000 volts, the separation of the rutile quartz mixture was tested at 0 hertz (direct current) to 2,500 hertz. The results are shown in figure 8. Rutile recovery peaked at 450 hertz and slowly declined as the electrical frequency increased. The rutile grade, however, ranged from about 30 to 60 percent without much correlation to the electrical frequency. Accordingly, the 450 hertz electrical frequency was selected as the optimum frequency.
Drum Rotation Speed & Feed Rate
The drum rotation speed of the separator is inversely related to the particles residence time between the two electrodes. The drum electrode rotation speed was varied from 6 to 75 rpm.
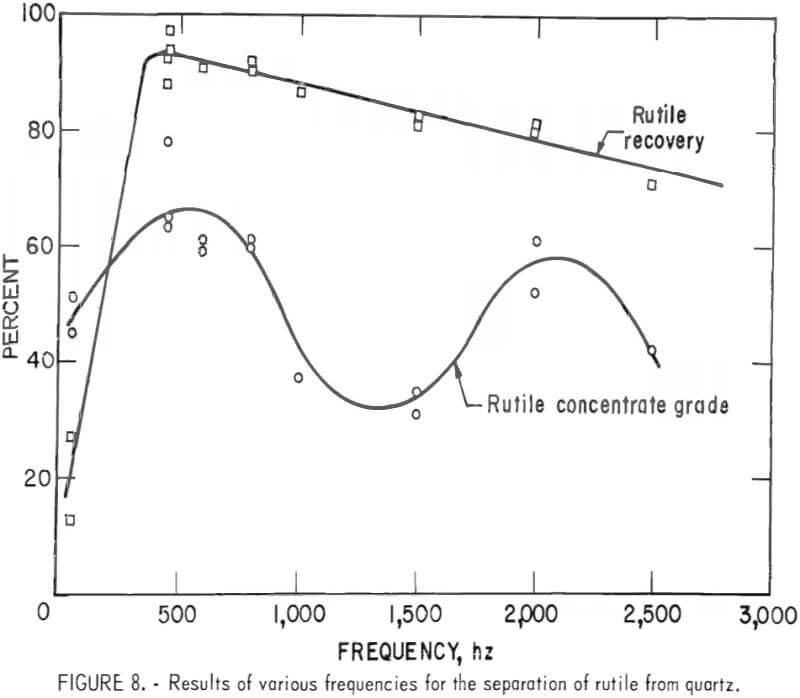
The separation responses, at residence times from 3 to 0.2 seconds, are shown in figure 9. The rutile grade dropped sharply at a residence time of less than 1.2 seconds. The rutile recovery began to decline for residence times less than 0.5 second. A 1.2-second residence time, corresponding to a drum electrode rotation speed of 13 rpm, was selected as the optimum.
During the preceding tests the feed rate to the dielectric separator ranged from 1 to 2 grams per minute at an 8 rpm drum speed. At 13 rpm, however, the feed rate was about 3 grams per minute. At both of these drum electrode speeds, the particle density on the drum electrode surface remained constant at about 60 grams per square meter, corresponding to about a third of a particle monolayer on the electrode surface. When the particle density was increased to about 100 grams per square meter or above, the rutile recovery appeared to drop significantly. Thus the best feed rate occurred with about 60 grams per square meter particle density, corresponding to about 3.0 grams per minute at a 13 rpm drum speed.
Power Consumption
Based on a feed rate of 3.0 grams per minute for the separator, the power consumption for dielectric separation at 1,000 volts was calculated. The electrical resistance through the separator, using a nitrobenzene and kerosene mixture with a K of 4.5, was measured at 330 megohms. The capacitance was calculated to be approximately 10 picofarads. At 450 hertz, the capacitive reactance of the separator was 220 megohms, indicating a power factor of 0.83. At 3.0 grams per minute and 1,000 volts, the electrical power consumption of the dielectric separator was 0.02 kilowatt-hours per tonne of feed. This does not include the power needed to rotate the drum electrode.
Optimum Design
Based on these characterization studies of the Bureau-developed rotating drum dielectric separator, the optimum design for dielectric separation of a 10-percent-rutile and 90-percent-quartz mineral mixture includes the following:
- A drum electrode with a 0.5-mm-diameter wire grid.
- A curved 20-mesh wire screen electrode.
- A nitrobenzone and kerosene mixture at a K of 4.5 with 1,000 volts of 450 hertz electricity.
- A drum electrode was rotated at 13 rpm, equivalent to a 1.2-second residence time for the particles.
- A feed rate of 3 grams per minute.
The rutile concentrate resulting from such a separator contained 62 percent rutile with a recovery of 94 percent of the rutile.
Various Sized Fractions
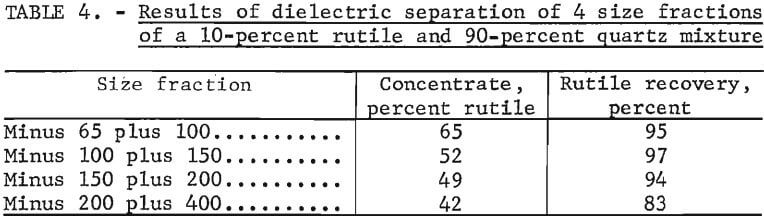
Four fractions of the rutile and quartz mixture sized from minus 65 to plus 400 mesh were tested with the dielectric separator. The results are shown in table 4. The grade decreased as the particle size decreased from 65 to 400 mesh.
Two Stage Separation
Two-stage dielectric separation of rutile from quartz was tested at the optimum operating conditions. The first pass through the dielectric separator with the rutile and quartz mixture produced a 68-percent rutile concentrate at 93-percent rutile recovery. A second pass of the concentrate through the dielectric separator produced a 92-percent rutile concentrate with 86-percent rutile recovery from the two stages and a low-dielectric product (middlings), which still contained about 23 percent rutile. Recirculating the middlings to the first-stage dielectric separator significantly increased the rutile recovery.
Dielectric Mineral Separation
Twenty-eight additional mineral mixtures were tested with the Bureau-developed rotating drum dielectric separator. Quartz or quartz combined with feldspar were the gangue minerals for the various mineral mixtures studied. The dielectric constants of quartz and feldspar varied from 4.0 to 5.0. Accordingly each mineral mixture was tested at dielectric values between 4.0 and 5.0 to determine the best separation dielectric constant.
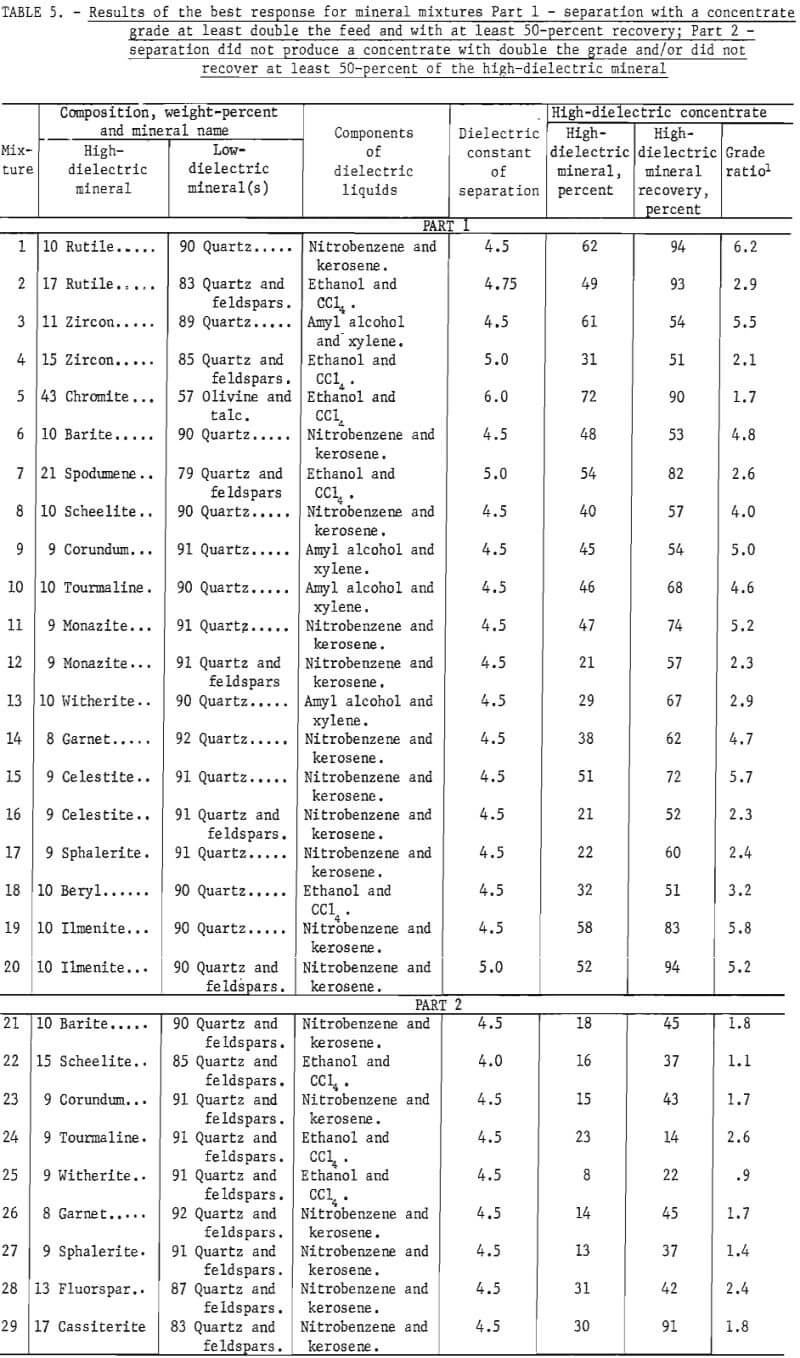
The results of these tests are summarized in table 5. The top portion of table 5 shows the best response for those mineral mixtures that were separated to produce a grade at least double the feed and with at least 50-percent recovery. The bottom portion of table 5 shows the best response obtained from the other mineral mixtures that did not recover better than 50 percent of the high-dielectric and/or did not double the grade of the high-dielectric mineral. Most of the mineral mixtures contained roughly 10 percent of the high-dielectric mineral. Unless otherwise indicated, the operating conditions for these studies were 1,000 volts of 450 hertz with a particle residence time of about 2 seconds, which was equivalent to an 8 rpm rotating drum electrode. The dielectric liquid and its dielectric constant are shown in the two tables.
Quartz or quartz-feldspars mixtures were the two low-dielectric products in nearly all of the mineral mixtures tested. The dielectric constant of the quartz and feldspars particles measured between 4.0 and 4.5.
The ratio of feldspars to free quartz was about 5 to 1. The 100-by 150-mesh size fraction was used in nearly all cases. For these tests three dielectric liquids, nitrobenzene-kerosene, ethanol-CCl4 , and amyl alcohol-xylene, at three dielectric constants between 4.0 and 5.0, were used. In general the high-dielectric mineral could be separated from the quartz, but it was much more difficult to separate from the quartz feldspars mixtures. The separations of minerals from quartz were greatest at a K of 4.5. The separations of the quartz-feldspars mixtures, in most cases, were greatest at a K of 5.0. The presence of the feldspars appeared to lower the concentrate grade and, in most cases, the recovery.
The minerals such as rutile, zircon, monazite, celestite, and ilmenite responded favorably to dielectric separation from the quartz and, to a lesser degree, from the quartz-feldspar mixture. Spodumene responded favorbly to the quartz-feldspars mixture. All of the other minerals tested were separable from the quartz, but did not respond to separation in the presence of the feldspars.
A sample of Montana chromite ore containing 43 percent chromite, 52 percent olivine, and about 5 percent talc was separated with the dielectric separator. A 72-percent-chromite concentrate was produced with 90-percent recovery of the chromite. The low-dielectric product contained 86 percent olivine.
Conclusions and Recommendations
The Bureau of Mines has designed and patented a separator for the continuous dielectric separation of minerals on a laboratory scale. The Bureau-patented dielectric separator beneficiated a 10 percent rutile and 90 percent quartz mixture to produce a 62 percent rutile concentrate that recovered 94 percent of the rutile. The optimum design factors include a wire grid on the surface of the drum electrode made with 0.5-mm-diameter wire, a curved 20-mesh wire screen electrode, a drum electrode rotation speed of 13 rpm (equivalent to a 1.2-second residence time for the particles), 1,000 volts at 450 hertz applied to the circuit, and a feed rate of 3 grams per minute (equivalent to a particle density of 62 grams per square meter of the drum electrode surface). The power consumption for this separation was 0.01 kilowatt-hour per metric ton of feed.
Nineteen of 28 additional mineral mixtures were adequately separated with the rotating drum dielectric separator. At least 50 percent of the high-dielectric mineral was recovered in a concentrate with at least double the grade from the feed. Dielectric separation of minerals has been demonstrated as a technological alternative to more conventional separation techniques. An economic evaluation of the method will determine its usefulness to the minerals industry.