Table of Contents
We recently prepared recommendations on methods of evaluating and classifying flammable liquids and solids for use in government transportation regulations. This report is the third in this series and describes the classification of oxidizing materials which are defined in the transportation regulations as substances that can yield oxygen readily to stimulate the combustion of organic matter. Since no classification test methods exist for these materials, procedures were developed by the Bureau for evaluating both solid and liquid oxidizers. The methods are designed to provide a relative measure of the increased ignition or burning hazard that may exist when inorganic oxidizers are mixed with an organic substance such as sawdust. They are not applicable to organic peroxides or to inorganic oxidizers that may detonate when heated with or without a combustible; other methods are being investigated where necessary for classifying these more hazardous substances.
Proposed Burning Rate Test for Solid Oxidizers
The development of a classification test method for inorganic oxidizers has received little attention compared to that given to organic oxidizers. Unlike the organic oxidizers, most inorganic oxidizers do not present a fire hazard when heated in the absence of a combustible material. Since wood or other cellulosic substances are likely to be present in a cargo fire, a burning rate test was developed for evaluating solid inorganic oxidizers with wood sawdust as the combustible. A select-grade red oak flooring was found to be suitable for this purpose; this same grade is used as a standard in the NFPA test method for evaluating the burning characteristics of building materials. The use of IBM card punchings as the reference combustible was also investigated; however, with an oxidizer such as ammonium perchlorate, the burning was erratic or not sustained because the oxidizer could not be distributed uniformly with the coarse-size combustible. Success was obtained in a few trials only by impregnating the card punchings with aqueous solutions of the oxidizer and subsequently drying them. Because of the complexity of this procedure and the limitations imposed by oxidizer solubility, the use of card punchings was abandoned.
Since the burning rate of sawdust can vary with the particle size and moisture content, it was necessary to control these variables to obtain reproducible results. The moisture content of the select-grade oak ranges from 6 to 9 percent. For the proposed test, the sawdust was initially screened to provide particles ranging in size from 12 to 50 mesh (Tyler screen series). The sawdust was dried in an oven at 215°±5° F for about six hours, and then test mixtures having various concentrations of the oxidizers were prepared. To obtain a uniform mixture, the materials were agitated for 10 minutes or more in a closed container. Generally, fine oxidizers were used “as-received” but coarse oxidizers were pulverized and screened to obtain samples at least as fine as the wood sawdust. For most of the oxidizers, a particle size range of about 20 to 100 mesh appeared to be adequate for determining their hazard classification by the proposed test. However, where the hazard level of such materials is uncertain because of particle size considerations, the burning rates of the mixtures should also be determined using oxidizer samples that have fractions finer than 100 mesh. The particle size range of most fine oxidizers in the present study was about 40 to 150 mesh.
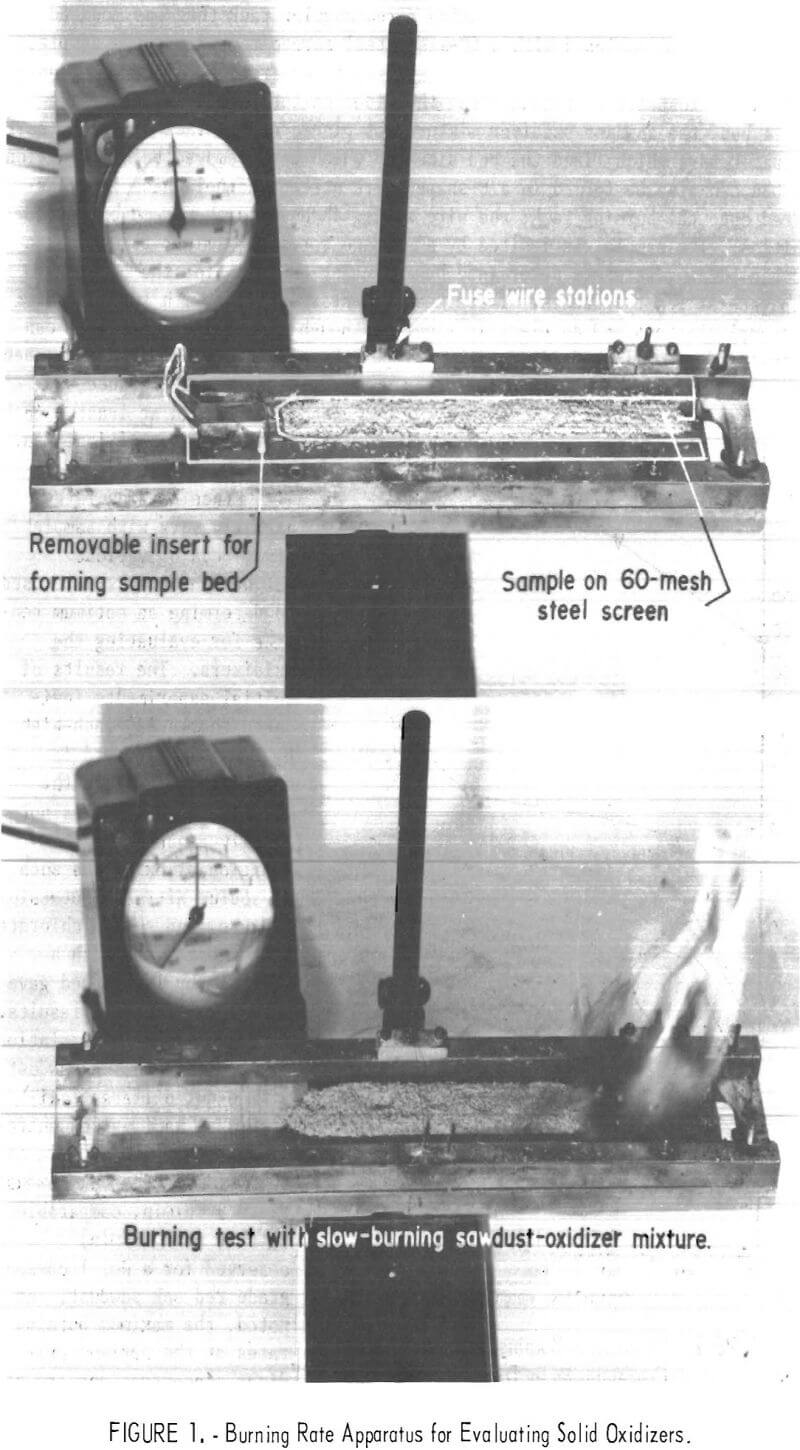
Burning rates were measured using a rectangular rack that was mounted horizontally and equipped with a 60-mesh steel screen to support the sample, as shown in figure 1. The sample bed was separated from the side rack mounts to insure unrestricted burning along the sides of the sample. To form the sample bed, the sawdust-oxidizer mixture was placed on the rack between a pair of spacer bars which fixed the bed size and which were removed before ignition. The bed can also be formed in a U-shaped wire screen channel which is transferred onto the burning rack; the wire screen channel is then removed before ignition. The sample was ignited by a propane torch or similar flame source and the burning rate was determined by measuring the time for the burning to propagate at least 5 inches. These measurements were made with two fuse wire (0.5 amp) stations and an electric timer, although slow-burning mixtures can be followed visually and timed with a stopwatch. In this work, the sample bed was normally 7 inches long and the rates were measured over a distance of 5 inches and at least 1 inch from the point of ignition.
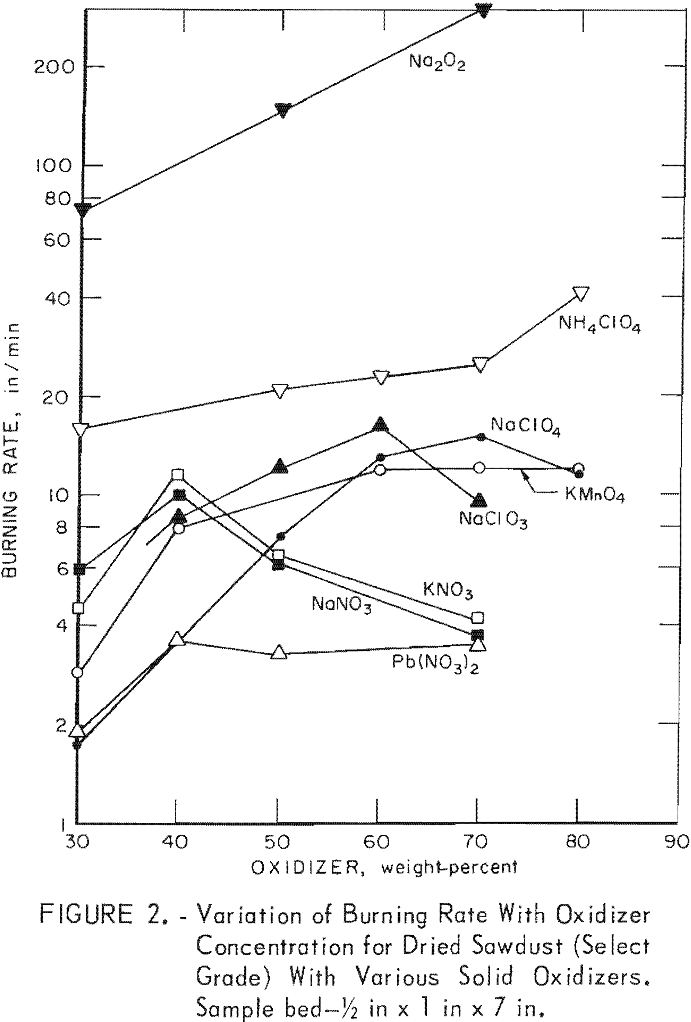
Since burning rates normally vary with sample size, beds of various heights and widths were used to determine an optimum bed size for evaluating the oxidizers. The results of initial experiments indicated that a ¼-inch-high by ½-inch-wide bed is unsatisfactory since the burning of sawdust was not greatly enhanced by the addition of oxidizers such as sodium nitrate, potassium nitrate, and sodium chlorate. Subsequent tests with a ½-inch by 1-inch bed gave much more favorable results. Figure 2 shows the variation of burning rate for sawdust with eight different oxidizers at various concentrations. The burning rate of the select-grade oak sawdust was 2.9 in/min, comparable to that (2.7 in/min) observed for a No. 1 common-grade red oak sawdust. As noted, the maximum burning rates at the optimum oxidizer concentrations are at least three times higher than sawdust, except in the case of lead nitrate. Furthermore, both the select-grade and No. 1 common-grade red oak sawdusts gave essentially the same burning rates in trials with 70-wt-pct concentrations of ammonium perchlorate (25 in/min vs 26 in/min) and sodium perchlorate (15 in/min vs 14 in/min). However, the rates with this bed size were lower than expected for some of the sawdust-oxidizer mixtures. For example, note the small differences (figure 2) between the values for sodium nitrate or potassium nitrate and lead nitrate at high oxidizer concentrations. Therefore, similar determinations were made with a larger sample bed, 1 inch high by 2 inches wide.
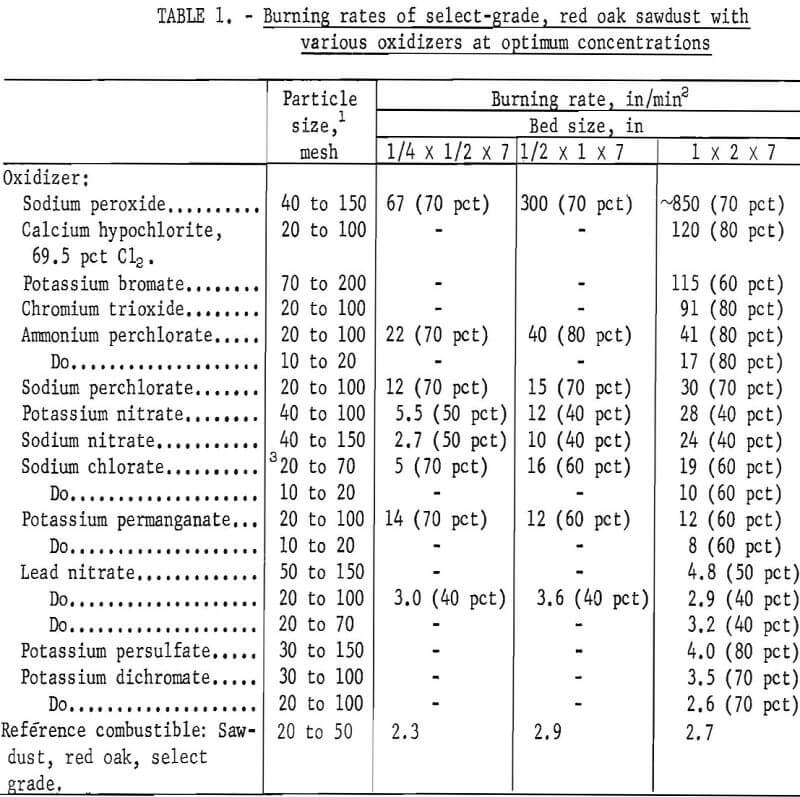
Table 1 lists the maximum burning rates obtained for 13 different oxidizers. Although the effect of bed size was not determined for all oxidizers, it is evident that the burning hazard can be predicted more reliably with a 1-inch by 2-inch bed than with the smaller beds. With the larger bed, the rates ranged from 3.5 in/min for potassium dichromate (30 to 100 mesh) to about 850 in/min for sodium peroxide (40 to 150 mesh), which was capable of spontaneously igniting wet sawdust. The listed values were the average of two or more trials and were reproducible to within ±10 pct for most materials. Typical examples using 1-inch by 2-inch beds are given below.
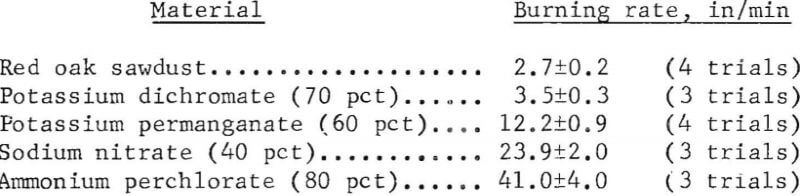
In most determinations, the oxidizer particle size was at least as fine as 20 mesh; a few oxidizers were also used at larger particle sizes for comparison. The noticeable effect that oxidizer particle size can have on the burning rates of the sawdust-oxidizer mixtures is shown in table 1 by the data obtained for ammonium perchlorate, sodium chlorate, and potassium permanganate. Since burning rates were relatively high (>10 in/min) with 20 to 70 mesh samples of the above oxidizers, finer samples of these materials were not evaluated, similarly, the particle size effect was not evaluated for other oxidizers which displayed a high burning hazard with the sawdust. In comparison, although relatively fine oxidizer samples were included in evaluating such oxidizers as lead nitrate and potassium persulfate, their burning rates were less than 5 in/min.
According to the data, the proposed test method permits classification of solid oxidizers into two or more groups based on their relative burning rates with a cellulose-type combustible such as wood sawdust. The least hazardous class includes those oxidizers that burn at low rates (<10 in/min) when mixed with the select-grade, red oak sawdust. A second class consists of oxidizers, such as the alkali nitrates and chlorates, which burn at relatively high rates (>10 in/min) when mixed with this sawdust. A third, more hazardous class should include those oxidizers, which when unmixed or mixed with a combustible, might ignite spontaneously and burn vigorously if moisture is present or if they are heated slightly. This class would include sodium peroxide and calcium hypochlorite (69.5 pct Cl2) which gave very high burning rates with the sawdust (table 1). A fourth class is also required for those oxidizers, such as ammonium perchlorate, which may detonate when heated under confinement or when exposed to shock.
Proposed Ignition Hazard Test for Liquid Oxidizers
Inorganic liquid oxidizers can increase the transportation fire hazard by exothermic reaction with other substances or by decomposition to products which ignite or sustain a fire. Generally, these liquids react with many organic substances and some are capable of producing spontaneous ignition when mixed with the combustible at normal or slightly elevated temperatures; some may also ignite spontaneously when heated in the absence of a combustible material, although such oxidizers are usually solids. Therefore, a test method was developed for evaluating the ignition or fire hazard of inorganic liquid oxidizers in the presence of a combustible such as the select-grade, red oak sawdust at various ambient temperatures. Wood sawdust was selected because it is highly reactive with many liquid oxidizers and representative of a combustible class that is likely to be present in the transportation of oxidizer cargoes. This method is not applicable to detonable liquid oxidizers, such as concentrated hydrogen peroxide (>90 pct) or perchloric acid (>72 pct). A shock sensitivity or thermal stability test is required for evaluating these types.
In the proposed test, the ignitability or reactivity of the oxidizer-sawdust mixtures was determined in an open reaction vessel using small quantities of the reactants. Although a maximum ambient temperature of 130° F is specified by the Department of Transportation for classification regulations, temperatures up to at least 190° F were used to compare the oxidizers, depending upon their reactivity. Such temperatures are not necessarily unrealistic, considering particularly the possibility of over-heating from the reaction of liquid oxidizers with contaminants. The reaction vessel in these experiments was a 200-cm³ Pyrex beaker that was equipped with insulated heating tapes and which rested on a flat ceramic heater; however, a stainless steel beaker can also be used. Because of possible violent reactions, the reaction vessel was placed in a larger vessel of heavy-duty steel and the experiments were performed in a protected area.
In a trial, a predetermined quantity of the sawdust (12 to 50 mesh) was added to the reaction vessel and brought to the desired temperature. The liquid oxidizer was then cautiously injected with a long hypodermic syringe (≥12 inches) from behind a protective shield, and the extent of reaction was determined from continuous temperature measurements and visual observations. The mixture temperature was measured with a 30-gage iron-constantan thermocouple protected against corrosion by a thin-walled glass sheath and located near the center of the reacting mass. Ignitions were confirmed visually since the flame reactions did not necessarily occur in the immediate area of the thermocouple; in many ignitions, the sawdust-oxidizer mixture was scattered or the flames occurred primarily near the top or outside of the test vessel. Generally, evidence of ignition was observed for periods up to at least 15 minutes. If no significant temperature increase occurred, experiments were made at higher temperatures and with various sawdust-oxidizer quantities. Preliminary trials are always made with a small quantity of oxidizer (<1 ml), particularly in the case of an oxidizer of unknown reactivity.
The proposed experimental procedure must be modified for evaluating the few volatile oxidizers which may be included in this hazard class by the Department of Transportation. Volatile oxidizers like nitrogen tetroxide, which boil near room temperature, are cooled to 32° F and samples of the liquid are injected into the reaction vessel by a hypodermic syringe. Oxidizers having very low boiling points, such as chlorine trifluoride, are injected directly into the test vessel as a gas. However, the vessel must be equipped with a cover to contain the gas. A loosely held glass cover or a diaphragm of low bursting pressure (-1 psig) is suitable for this purpose.
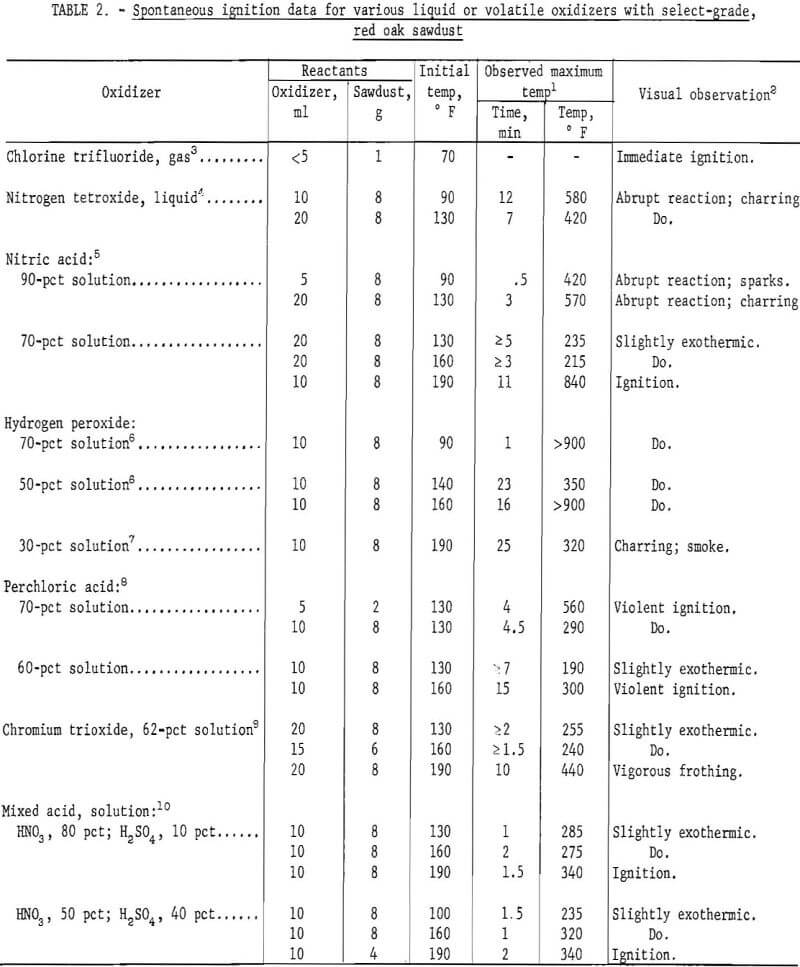
Table 2 compares selected results from the ignition experiments with several liquid or volatile oxidizers and the red oak sawdust. These results indicate the spontaneous ignition hazard of the various mixtures at initial temperatures up to 190° F, depending upon the oxidizer composition. Most of the results were obtained with 8 g of sawdust and 10 to 20 ml of oxidizer. Other oxidizer quantities were also used, although they were not necessarily more favorable for reaction. Of the oxidizers investigated, chlorine trifluoride and 70 pct hydrogen peroxide displayed the greatest spontaneous ignition hazard with the wood sawdust at an ambient temperature of 90° or 70° F, The sawdust was ignited immediately upon contact with the chlorine trifluoride vapors, and after one minute when mixed with a 70-pct hydrogen peroxide solution; these mixtures burned vigorously following ignition. Nitrogen tetroxide and 90 pct nitric acid also displayed considerable reaction with the sawdust at room temperature. These oxidizers produced abrupt reactions and extensive charring, as well as sparks in the trial with 90 pct nitric acid, but normal ignitions were not detected and the mixtures burned rather mildly when ignited with a flame; however, the charred mass produced with each oxidizer inflamed and burned vigorously upon further addition of the oxidizers. The most violent ignitions and most vigorous burning occurred with perchloric acid- sawdust mixtures, which ignited at 130° F (70 pct) or 160° F (60 pct), Other oxidizers such as 70 pct nitric acid, 50 pct hydrogen peroxide, and two mixed acid solutions (HNO3 + H2SO4) appeared to require temperatures between 140° and 190° F for igniting the sawdust. The least reactive oxidizers by this test method were 30 pet hydrogen peroxide and 62 pet chromium trioxide (“chromic acid”) solutions.
To compare the reliability of the present method with that of other possible methods, hypergolicity experiments were performed with several oxidizers using isopropyl alcohol as the fuel. The experiments were performed in a test tube at room temperature by injecting the fuel into the oxidizer, or the oxidizer into the fuel, at various fuel-oxidizer ratios. However, although ignitions occurred with 90 pct nitric acid, little reaction resulted with 70 pct hydrogen peroxide or 70 pct perchloric acid. Similarly, results were not promising using turpentine, which is hypergolic with nitrogen tetroxide. Since a fuel that may be suitable for comparing the hypergolic behavior of various oxidizers may not be realistic for evaluating their transportation fire hazard, further hypergolic ignition work was not pursued.
The test procedure described for evaluating liquid oxidizers was also used without the sawdust to examine the spontaneous decomposition hazard of two solid oxidizers, calcium hypochlorite (69.5 pct Cl2) and sodium dichloroisocyanurate. The latter substance is classified as a combustible and an oxidizing agent. To reduce heat losses during reaction, the samples (10 g) were surrounded by glass wool in the 200-cm³ reaction vessel. At an initial temperature of 160° F, the maximum temperature from decomposition was between 220° and 240° F for both materials and the duration of each test was over 30 minutes. At 190° F, the calcium hypochlorite decomposed rapidly after 4 minutes and produced a maximum temperature of 650° F. In comparison, the sodium dichloroisocyanurate showed accelerated decomposition at a temperature of about 240° F. The heating duration was 18 minutes and the maximum temperature was greater than 900° F. Although these results indicate the relatively high decomposition hazard in heating such oxidizers, an adiabatic test method is necessary to determine their decomposition temperatures more precisely.
Classification of Oxidizers by Proposed Test Methods
The test methods described in this report should be suitable for classifying the transportation fire hazard of most inorganic oxidizing materials. The greatest number of such hazardous substances are solid oxidizers, and most of them can be evaluated by the horizontal burning rate test proposed with select-grade, red oak sawdust as the reference combustible. Liquid oxidizers can be evaluated largely by the proposed spontaneous ignition test using the same combustible as in the burning rate test. For those liquids that display a low fire hazard with such a combustible, but which are considered highly corrosive, the latter reactivity hazard should be used for their classification. A test for evaluating the corrosive hazard is beyond the scope of this report. The most hazardous class of inorganic oxidizers are those that may detonate with or without a combustible and which requires special thermal or shock stability tests for their evaluation. Possible test methods for classifying organic peroxides are being investigated by the Bureau.
Using the proposed test methods, the following hazard classification for inorganic oxidizing materials is recommended:
Class 1: Oxidizing materials which when mixed with dried red oak sawdust (12 to 50 mesh) can be ignited by an external source but burn at low rates (<10 in/min) in exposed horizontal beds at least 1 inch high and 2 inches wide.
Class 2: Oxidizing materials which when mixed with dried red oak sawdust can be ignited and burn at high rates (>10 in/min) in exposed horizontal beds at least 1 inch high and 2 inches wide. This class would also include liquid oxidizers which can ignite the sawdust spontaneously when mixed and heated slightly (<200°F) in an open container, but which produce only moderate burning.
Class 3: Oxidizing materials which when mixed with dry or wet red oak sawdust in an open container can ignite spontaneously at normal or slightly elevated temperatures (< 200° F) and which produce vigorous burning. This class would also include oxidizers that are capable of violent, self- sustained decomposition when heated or exposed to flame.
Class 4: Oxidizing materials which when unmixed or mixed with a combustible may detonate if heated or subjected to shock.
Table 3 compares the hazard ratings by the proposed classification criteria for the various oxidizers investigated in this study and for several other oxidizers whose ratings were defined from available information on their reactivity.10 11 An NFPA “Oxidizing Materials Subcommittee” has prepared a tentative classification system for oxidizers but this has not been published yet. The latter system is somewhat similar to the Bureau’s; however, the NFPA system as presently proposed appears to be less discriminating between some solid oxidizers which can greatly increase the burning hazard and those which are considered least hazardous. The Inter-Governmental Maritime Consultative Organization (IMCO) has also defined a classification system for oxidizers, as cited in reference 11; its usefulness is somewhat limited since various hazard levels are not specified for inorganic oxidizers (class 5.1) or organic peroxides (class 5.2) that are listed as “oxidizing substances.”

Under the Bureau’s classification, the most hazardous class (class 4) would include such oxidizers as ammonium perchlorate, hydrogen peroxide solutions with concentrations over 90 wt pct, and perchloric acid solutions with concentrations over 72 wt pct, all of which can be detonated. These materials are assigned to a similar hazard category under the newly proposed NFPA classification for oxidizing materials. The next most hazardous class (class 3) includes chlorine trifluoride, 70-pct hydrogen peroxide solutions, and 60- to 72-pct perchloric acid solutions which are highly reactive and burn vigorously when mixed with red oak sawdust at normal or slightly elevated temperatures. These materials are designated as “corrosive liquids” in recently revised government transportation regulations but should be classified as “oxidizing materials” in view of their extremely high fire hazard with cellulosic combustibles; this recommendation is consistent with that of other investigators. Nitrogen tetroxide and 90 pct nitric acid, which can be extremely hazardous with cellulosic materials as well as with many hydrocarbon fuels, are also assigned to this category. Other oxidizers listed under class 3 are calcium hypochlorite (69.5 pct Cl2) and sodium dichloroisocyanurate which are capable of violent decomposition upon moderate heating. Sodium peroxide is also listed here because of its high spontaneous ignition hazard when mixed with moist sawdust and the high burning rates that can result with wet or dry sawdust mixtures.
The greatest fraction of solid and liquid oxidizers will tend to fall into the last two hazard classes (class 1 or 2 ) of the proposed classification system. Such solid oxidizers as chromium trioxide, potassium bromate, and the alkali metal nitrates, chlorates, and perchlorates are typical of the class 2 materials, which greatly increase the burning rate of the reference combustible. Liquid oxidizers in this class include 50 pct hydrogen peroxide, 70 pct nitric acid, and nitric acid-sulfuric acid (nitrating acid) solutions. Here again, it is worth noting that the acid solutions and the peroxide solution are classified as “corrosive liquids” in the transportation regulations; these should also be described as “oxidizing materials” because of their relatively high reactivity or ignitability hazard with combustibles like the red oak sawdust. According to the present classification scheme, a distinction is made between the alkali metal nitrates and the alkaline-earth or heavy metal nitrates which present a lower degree of fire hazard with the wood sawdust combustible. As noted, the latter oxidizers are assigned to the least hazardous class (class 1). This class also includes such solid oxidizers as potassium persulfate and potassium dichromate and such liquid oxidizers as “chromic acid” and weak hydrogen peroxide (30 pct) solutions; a “corrosive liquid” classification would appear appropriate for these liquid oxidizers. Since potassium dichromate has recently been removed from the IMCO class (5.1) for hazardous inorganic oxidizers, large-scale burning tests should be made using the red oak sawdust to determine whether a lower hazard ranking is justified for this oxidizer, as well as for any other oxidizer whose classification may be questionable.
In the application of the proposed test methods, it must be recognized that a reliable hazard rating may not be possible for all oxidizers using a single reference combustible. If the packaging material is not cellulosic in nature, it is conceivable that an oxidizer may display a greater level of hazard than observed with the select-grade, red oak sawdust used in the present study. Therefore, if it is suspected that a greater fire hazard exists due to the nature of the packing material, tests should be made with that material as the combustible for comparison. In the event the hazard class of an oxidizer appears to be borderline by the proposed criteria, the class reflecting the greater hazard should be used to define its classification.
