Nitrate is normally present in waters associated with mining as a result of blasting activities using ammonium nitrate or dynamite. It may also be present in groundwater and surface water in agricultural areas from fertilizer use. Nitrate is also normally present in municipal and industrial waste streams after the aerobic degradation of ammonia.
The concentration of nitrate in water is of primary concern due to potential human health and environmental impacts from groundwater or surface water usage. The toxicity of nitrate to humans is due to the body’s potential conversion of nitrate to nitrite, particularly in infants. Elevated nitrite levels can cause reduced oxygen concentrations in the bloodstream, a condition known as methemoglobinemia. Accordingly, the maximum allowable regulatory limit for nitrate is 10 mg/L as nitrogen. Discharge of nitrate containing waters to surface water is also an environmental concern because, as a limiting nutrient, nitrate potentially can produce undesirable aquatic plant growth.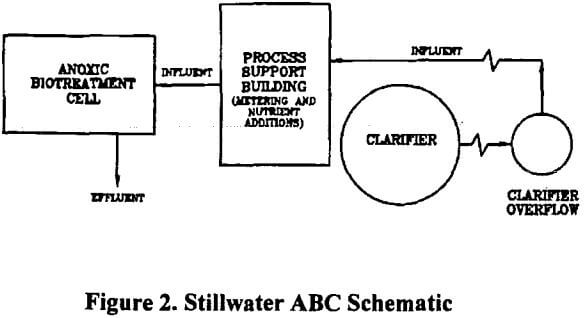
Numerous technologies have been developed and used as a means of removing nitrate from water. Ion exchange (IX) and reverse osmosis (RO) have been successfully used worldwide for over 20 years to remove nitrate and other ions to low concentrations, but their primary disadvantages are high capital and operating costs, and generation of a concentrated waste stream. Conventional biotreatment systems have also been used for many years, primarily in municipal wastewater treatment plants. These technologies include sequencing batch reactors, rotating biological contactors, and packed-bed and fluidized-bed systems. While costs for conventional biotreatment systems are generally lower than those for IX or RO, costs are still substantial and a biomass waste stream must be handled. Nitrate, like other constituents such as arsenic, sulfate, thallium and selenium, is a common parameter in mine waters which requires an innovative, cost-effective treatment technology. Industry, particularly the mining industry, has been searching for a simple, effective system for nitrate removal such as the Anoxic Biotreatment Cell (ABC).
Anoxic Biotreatment Cell ABC Process
The ABC technology uses biological methods to remove nitrate from effluent waters, and was developed and tested in mining operations. This process employs several key features which make it a significant advance in technology. Based on bench-scale testing and production-scale operations, the ABC technology is very promising for treating a range of nitrate containing waters.
Denitrification
In the biological process called denitrification, aqueous concentrations of nitrate are converted to harmless nitrogen gas. Nitrogen present in the form of dissolved nitrate may be removed from water through the action of denitrifying bacteria (DNB), which convert the nitrate to nitrogen gas (N2). DNB are facultative anaerobes, meaning they reduce oxygen preferentially over nitrate and will reduce nitrate only when oxygen is not readily available. Therefore, microbial nitrate reduction can only occur in environments which are anaerobic (no oxygen) or anoxic (low oxygen concentration).
An external carbon source such as methanol is often added to enhance denitrification. The carbon source serves as an electron donor (is oxidized to CO2) while nitrate acts as the electron acceptor (is reduced to N2). The reaction in which methanol (CH3OH) supplies electrons to produce energy is called dissimilatory nitrate reduction:
6 NO3- + 5 CH3OH → 5 CO2 + 3 N2 + 7 H2O + 6 OH-………………………………….(1)
Methanol is also required to supply the carbon for creating new microbial cell mass (abbreviated as the formula C5H7O2N) in the reaction, called assimilatoiy nitrate reduction. The overall reaction, combining dissimilatory and assimilatory nitrate reduction (EPA 1993), is:
NO3- + 1.08 CH3OH + 0.24 H2CO3 → 0.056 C5H7O2N + 0.47 N2 + 1.68 H2O + HCO3-……….(2)
When converted to a mass basis, about 2.5 g of methanol are required to reduce 1 g of nitrate (as nitrogen) to nitrogen gas. The nitrogen and carbon dioxide gases produced in the reactions are typically in excess of the amounts which are soluble in water, which requires that the bioreactor have a venting system. Nutrients such as phosphate are also required for bacterial growth, but in concentrations too low to be shown in Equation (2).

