Table of Contents
The Bureau of Mines Twin Cities Research Center conducts research to assist in solving problems that occur during solution mining of metals. Solution mining of uranium involves injecting a carbonate-bicarbonate (basic) or sulfate (acidic) leach solution through injection wells into an ore body where the lixiviant dissolves the uranium from the ore. Uranium bearing lixiviant is then brought to the surface through production wells and is recovered by ion-exchange methods. In a similar manner, copper can be leached from its host rock with sulfuric acid and is recovered from the lixiviant at a surface unit operation. Plugging of the injection wells is often a problem during solution mining.
Plugging may be caused by improper well construction, invasion by solid particles from drilling fluids or cementing operation, precipitation of chemical salts, bacterial effects, fine particles in the injection fluid, and/or clay swelling and dispersion. Acid or water jet perforation can be used to stimulate wells that exhibit high resistance to injection but the treatments are not universally applicable and the benefits are generally temporary.
Uranium is most commonly leached from sandstone host rocks, and certain of these formations are water sensitive. Such formations are susceptible to permeability damage by exposure to introduced water that has a chemical composition different from the natural, interstitial water. The introduced water can upset the swelling equilibrium of the clay-water system.
A formation’s susceptibility to permeability damage is related to the salinity of the water to which it is exposed and to the type and amount of clay mineral constituents present. Reduction in salinity of interstitial water can cause clay swelling, which may plug pores and reduce permeability. Swelling also causes the clay platelets to break up into finer, negatively charged particles. These negatively charged particles will repel one another and, consequently, disperse through the interstitial fluid until they lodge in constrictions in permeability channels, thereby plugging the channels.
The common swelling-clay minerals are the montmorillonites, mixed-layer clays, and certain types of illite. Clay particles in their natural state are at equilibrium with the saline water which usually occurs in the formations. Swelling occurs when fresh water from drilling or leaching activities replaces the saline water in the formation. In general, the amount of swelling increases with a decreasing in salinity of the injected water. Therefore, a concentrated brine would cause the least damage and fresh water the most damage. The nature of the clays is also important. Clays in the calcium form do not disperse as easily in low-salinity water as clays in the sodium form. It is thought that sodium clays dissociate in low-salinity water, creating sodium ions and clay particles with a net negative charge high enough to cause the particles to repel one another and thus be dispersed.
The form of a clay can be altered by flowing a solution through it. Through cation exchange, a calcium clay may be changed to a sodium clay by passing a concentrated sodium-bearing solution through it. The cation-exchange capacity of the clay minerals is caused by broken bends, substitution within the lattice structure, and replacement of the hydrogen of exposed hydroxyls by the exchangeable cation. It is thought that if at least one-tenth of the dissolved salts in the water are magnesium and calcium, swelling and dispersion of clays will be minimal. Hydrated calcium and magnesium ions seemingly restrict the amount of adsorbed water on the clay particle to a well developed configuration of minimal thickness, while sodium ions allow oriented water to grow to very great thicknesses on the clay particle.
For the above reasons, uranium solution mining operators often use native ground water to prepare the leach solution because it usually contains calcium and magnesium ions. However, the leach solution may still cause changes in formation water chemistry great enough to cause the clay to swell, and if the clay is in the swelling or dispersing form, any fluid flowing through the formation will result in permeability damage.
Commercial clay stabilizers have been developed to reduce permeability loss caused by clay swelling and dispersion. Inorganic clay stabilizers that are available include hydroxy aluminum and zirconium oxychloride solutions.
The higher charge cations of these clay stabilizers will adsorb on the clays more readily than monovalent or divalent cations because the attractive force between the negatively charged clay particles and the stabilizer cations is exponentially related to the charge on the cations. These clay stabilizers have been shown to be effective in reducing permeability losses, but removal of these cations from the clays may occur when and if the wells undergo acid treatment thereby reducing their effectiveness. A series of acid-resistant, organic cationic polymers have also been developed to prevent permeability loss under a broad range of pH conditions.
Organic cationic polymers posses features which suggest potential usefulness for reducing the permeability losses often occurring during solution mining. Tests were conducted at Bureau facilities to define the range of pH conditions under which their use would be appropriate and to determine their effectiveness in preventing permeability loss. A hydrocarbon polymer with a nitrogen nucleus was chosen for laboratory testing. This report describes laboratory permeability leaching tests conducted on three clayey ores (two uranium and one copper), employing both alkaline and acidic leach solution. Clay-swelling tests and zeta potential measurements were also conducted to determine the fundamentals of permeability improvements.
Equipment and Materials
Permeameter
Permeability is a measure of a porous medium’s capacity for transmitting a fluid and consequently, is a key parameter in determining the ability of an ore formation to accept leaching solution through injection wells. A permeameter was constructed and accessories were assembled in order to compare permeabilities of treated and untreated formation samples under controlled laboratory conditions. The permeability test apparatus (fig. 1) includes a confining pressure cell, equipment for supplying a compressive load to the sample, and a means to pressurize the influent leach solution reservoir. The cell is a 5-cm-ID stainless steel cylinder with opposing stainless steel platens (pistons) employed to contain the 250 g sample and test liquids under compression. This apparatus permits metals to be leached from the sample while simultaneously measuring permeability.
A large plexiglass column 15 cm in diameter and 80 cm long, was used as a constant-head permeameter for leaching larger (35-kg) samples of unconsolidated ore.
Ore Samples
Two of the three materials used in this investigation were low grade uranium-bearing sandstones from Wyoming: One from the Bear Creek Mine, 21 miles northeast of Casper in the Powder River Basin; the other from a pit in the Red Desert of Wyoming. The third sample was a clayey copper ore from the Lakeshore Mine by Noranda Copper Co. near Casa Grande, AZ.
Particle size distribution of the Bear Creek ore is shown in table 1. The minus 200 mesh material was primarily calcium montmorillonite. The plus-200 mesh material was primarily quartz with smaller amounts of feldspar, mica and calcite.
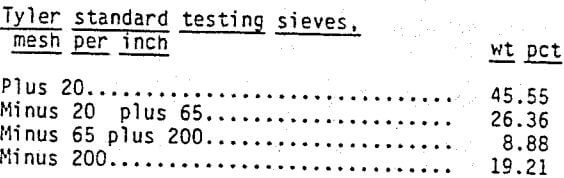
The size distribution of ore from the pit in the Red Desert is shown in table 2. The minus 200-mesh material was primarily calcium montmorillonite while the plus 200-mesh material was composed of quartz and feldspar with smaller amounts of mica and calcite.

The clayey copper ore from the Lakeshore Mine was crushed and screened to minus 4-mesh. The crushed feed contained 13 wt pct minus 200- mesh material. The ore was a porphyry granite composed of quartz, potassium feldspar, albite, muscovite, kaolinite, chlorite, and chrysocolla. Small amounts of martite, goethite, and manganese oxides were also present.
Leach Solutions
Common solutions used for solution mining of uranium ore are prepared from ammonium carbonate-bicarbonate, sodium carbonate-bicarbonate, carbon dioxide, and sulfuric acid. Oxidizer such as oxygen, hydrogen peroxide, or sodium chlorate are employed to convert uranium from its insoluble +4 valence state to the soluble +6 valence state.
The cation associated with the carbonate-bicarbonate anions does not affect leaching reactions but can affect ore permeability. As previously pointed out, sodium leach interacts with the clay to cause swelling and dispersion. Although sodium carbonate-bicarbonate can cause permeability loss, its low cost favors its use when clay is not present and would make it an attractive choice for many operations if a method such as the proposed application of cationic polymers would prevent clay breakup and dispersion. Tests with both sodium bicarbonate leach near pH 8.5 and sulfuric acid leach near pH 2 were made to evaluate the stabilizing effects of a cationic polymer.
Organic Cationic Polymer Clay Stabilizer
A commercial cationic polymer (Cla-Sta-B) was chosen for testing which was likely to function under both acidic and basic pH conditions. The polymer is a water-soluble cationic hydrocarbon comprised of long-chain molecules with attached nitrogen atoms, some of which are quaternary. Polymers of this type can have very high molecular weights often exceeding 100,000. A number of diluents or carriers are used to make the clay stabilizing polymer more water soluble. Among these are ammonium chloride (2 pct), sodium chloride (8 pct), hydrochloric acid (5 to 15 pct), potassium chloride (4 pct), and methyl alcohol (5 pct). Although ammonium chloride is the most efficient diluent of these, the environmental problems associated with the ammonium ion dictate that an alternate diluent be selected for solution mining applications. Potassium chloride was the diluent employed with the polymer during laboratory permeability-leaching tests.
Experimental Procedure Results and Discussion
Permeability-Leaching Tests
Simultaneous permeability and leaching tests on 250-g samples of Bear Creek uranium ore were conducted in the confining pressure cell apparatus using both basic and acidic leach solutions. A 30-kg/cm² compressive load was applied and maintained on the sample while leach fluid was forced through it under a measured pressure gradient, which was regulated in order to maintain a flow rate of approximately 1 pore volume per hour.
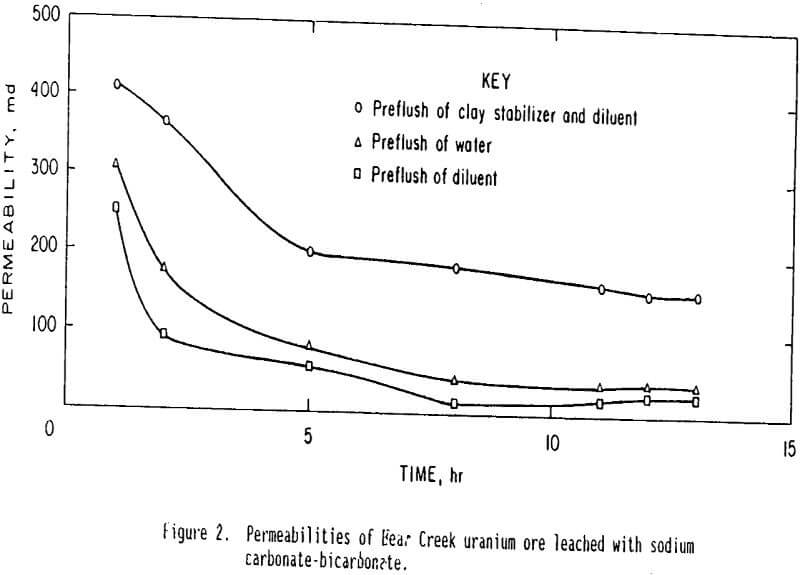
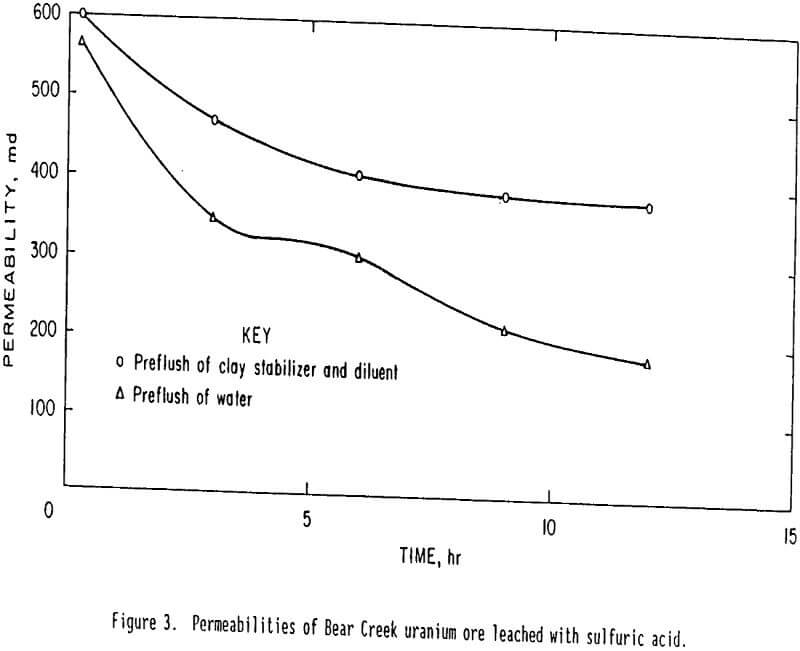
Tests with sodium bicarbonate leach solutions were made after pretreatment with three types of solutions: (1) 2 pct cationic polymer and 4 pct potassium chloride in tapwater, (2) tapwater, and (3) 4 pct potassium chloride in tapwater. Tests with sulfuric acid leach solutions were made after pretreatment with two types of solutions: (1) 2 pct cationic polymer and 4 pct potassium chloride in tapwater and (2) tapwater. In each instance the sample was allowed to retain the pore volume of preflush fluid for ½ h before leaching with sodium carbonate-bicarbonate or sulfuric acid leach solutions. Effluent samples were taken and permeability was determined at intervals during the tests. The leach solution was not recycled in either case. Results of the sodium carbonate-bicarbonate leach tests on Bear Creek uranium ore are shown in figure 2. Results of the sulfuric acid leach tests on this ore are shown in figure 3. Points on the curves represent the average of two tests with each type of preflush. Results from the sodium carbonate-bicarbonate leaching indicate that, after 12 h of leaching, the ore pretreated with the clay stabilizer had a permeability about five times higher than that of the untreated (tapwater pretreated) ore. These results also confirm that the potassium chloride diluent does not prevent permeability loss but actually causes some additional permeability loss. Therefore the increased permeability is caused by the stabilizing cationic polymer. Results from sulfuric acid leaching indicate, that after 12 h of leaching, the ore pretreated with the clay stabilizer had a permeability about twice that of the untreated (tapwater pretreated) ore. Comparison of the results in figure 3 and those in figure 2 indicates that permeability loss is not as great for the sulfuric acid leach as the sodium carbonate-bicarbonate leach. After 12 h of leaching, the permeability is about 380 md for sulfuric-acid-leached ore and only about 150 md for sodium-carbonate-bicarbonate-leached ore.
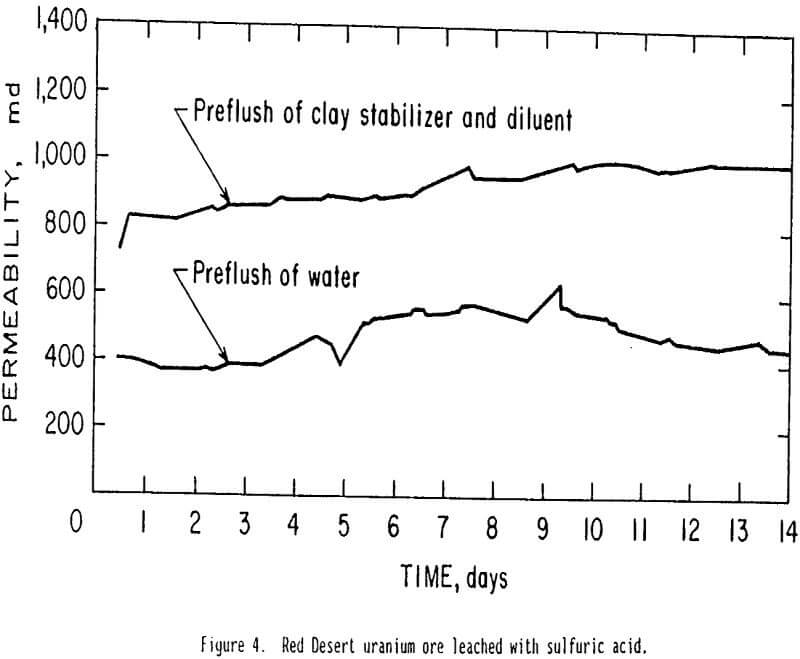
The Red Desert of Wyoming ore was leached in the large constant-head permeameter using sulfuric acid solution. One test was made after a preflush of 2 pct clay stabilizing cationic polymer and 4 pct potassium chloride in tapwater. A second test was made after a preflush of tapwater alone. The preflush solutions were bottom-fed into the column through the outlet and were allowed to rise to the constant fluid level 155 cm above the outlet. Sulfuric acid leaching began one half hour after the preflush. Results are shown in figure 4 and indicate that the cationic polymer treated ore had a permeability twice as great as the untreated (tapwater preflush) ore throughout the 14-day test. These results further confirm that the cationic polymer could be used to reduce permeability losses when uranium ore is leached with acid.
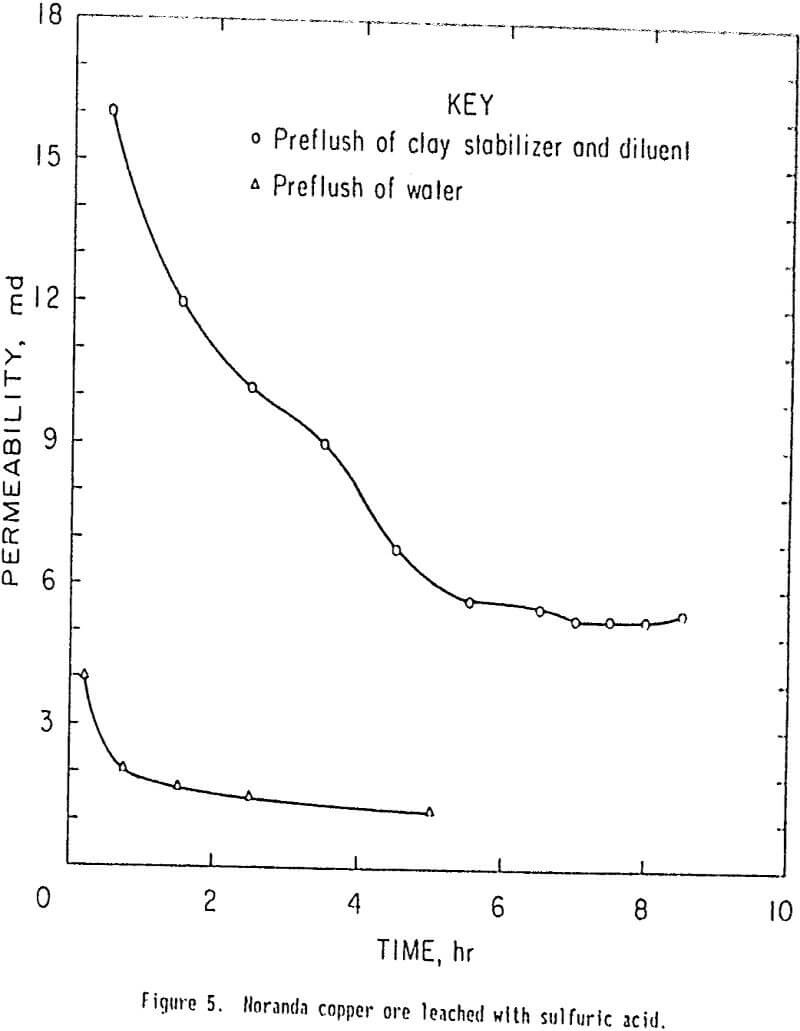
Permeability-leaching tests were also made on 250-g samples of the clayey copper ore in the confining-pressure-cell apparatus with a sulfuric acid leach solution of pH 2. These tests were conducted to determine the effect of cationic polymer on the permeability of a clayey copper ore rather than a uranium ore. One test was made after a preflush of 2 pct cationic polymer and 4 pct potassium chloride in tapwater, and the other test was made after a preflush of tapwater alone. The sample was allowed to retain the pore volume of preflush fluid for ½ h before leaching with sulfuric acid solution. Results of these tests are snow in figure 5 and indicate that after 9 h the permeability of the cationic polymer treated ore was five times greater than the permeability of the untreated (tapwater-pretreated) ore. The permeability had stabilized at about 1 md in the tapwater pretreatment test. Consequently, the test was terminated at 5 h.
It was assumed that if the test was continued, the permeability would be about 1 md it the end of 9 h. Subsequent large-scale column leaching tests on treated and untreated ore conducted by Noranda have confirmed these results, and show that cationic polymer pretreatment reduces the permeability loss in acid leaching of a clayey copper ore.
Effluent Analysis
The predominant clay in the uranium ores which were tested was a calcium montmorillonite. As described in the Introduction, calcium in the clay lattice can be replaced by sodium ions in the leach solution. This results in a clay form that is more likely to swell and disperse. An analysis of calcium and sodium in the effluent from tests using sodium carbonate-bicarbonate leaching solution demonstrated that as leaching proceeds, calcium is in fact displaced by sodium and appears in the effluent as a function of time, if there were no exchange of sodium ions for calcium ions in the montmorillonite of the Bear Creek ore, the sodium content of the effluent would be about 650 to 700 mg/L while the calcium content of the effluent would be near zero. However, after 1 h of leaching with NaHCO3-NaCO3, the calcium content is about 400 mg/L and the sodium content is about 280 mg/L, thereby proving that sodium ions have displaced calcium ions in the clay. As leaching time increased, the clay became more saturated with sodium ions, fewer calcium ions are displaced, and therefor fewer calcium ions appear in the effluent. The exchange of sodium ions for calcium ions in the clay fraction during sodium carbonate-bicarbonate leaching also explains why there is a sharper drop in permeability for untreated samples leached with sodium carbonate-bicarbonate compared with the drop in permeability for untreated samples leached with sulfuric acid. This has also been reflected in actual in situ leaching experience.
Clay-Swelling Test
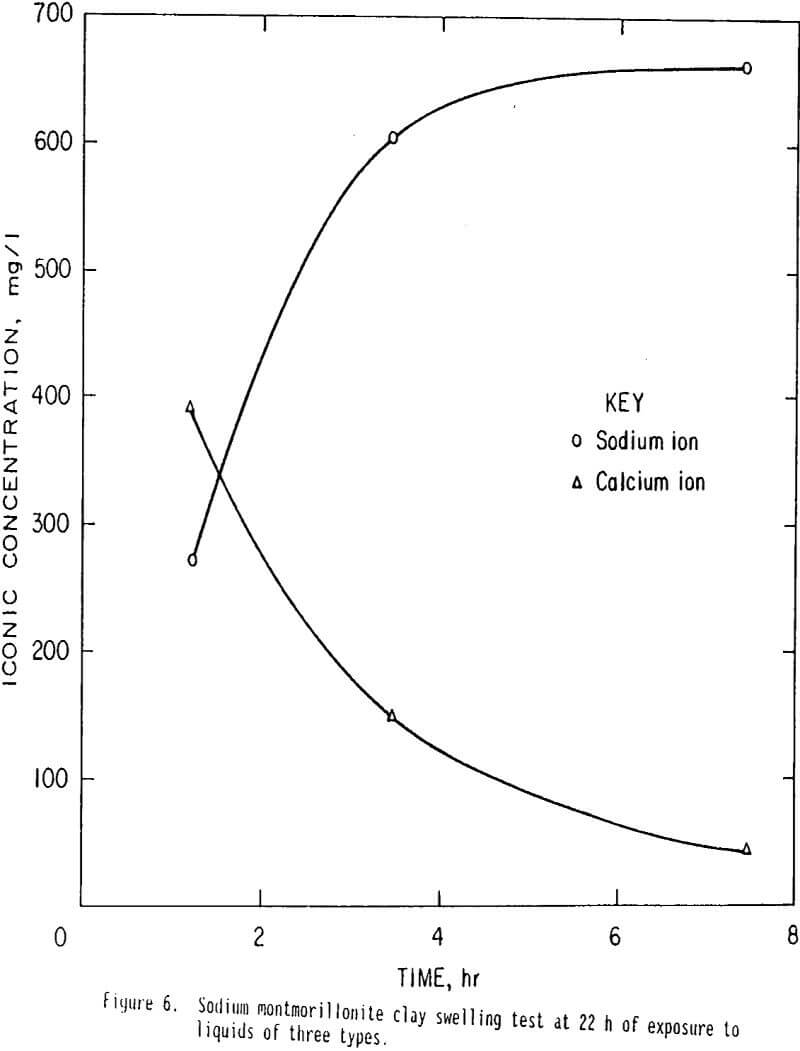
Tests were conducted to evaluate the effectiveness of the cationic polymer in preventing clay swelling. Three vials, each containing 10 grams of sodium montmorillonite were each exposed to different solutions to evaluate the effectiveness of the cationic polymers ability to prevent clay swelling. Results are shown in figure 6. Clay in vial 1 was exposed to sulfuric acid leach solution of pH 2. Clay in vial 2 was exposed to acid leach solution and 2 pct potassium chloride diluent. Clay in vial 3 was exposed to acid leach solution, 2 pct diluent, and 1 pct clay stabilizing cationic polymer. The test results demonstrate that after 22 h of exposure to the liquids, the height of clay in vial 1 was 47 units, height in vial 2 was 40 units, and in vial 3 the height was 30 units. Volume of clay in vial 3 which was treated with diluent and polymer before exposure to acid leach, was only 63 pct of the volume of clay in vial 1 (exposed to leach only), and 75 pct of the volume of clay in vial 2, (treated with diluent before exposure to acid leach solution). The cationic polymer and diluent reduce clay swelling 25 pct more effectively than diluent alone indicating the cationic polymer prevents clay swelling. This characteristic is important in retarding clay swelling when a sodium leach is used or when sodium montmorillonite is present in the ore body.
Zeta Potential Measurements
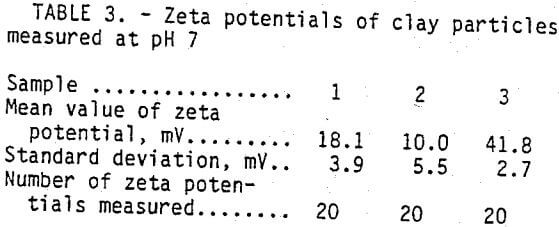
Zeta potential measurement were made on clay subjected to various treatments to provide additional clues regarding the mechanisms responsible for the clay stabilizing cationic polymers effect on the clay particles.
Electrophoretic mobility of finely divided, solid particles in an aqueous suspension can be measure by using a microelectrophoresis instrument. Electrophoretic mobility is the rate of particle motion in an electric field. The motion is brought about by the interaction of the electrostatic charge at the particle-medium interface and the imposed electric field. Electrostatic particle charge is expressed as zeta potential in units of millivolts. Three samples of finely divided clay particles were each exposed to different liquids prior to the measurement of zeta potential on the finely divided clay particles. Samples of sodium montmorillonite clay each weighing 2 g were blended into 1-L samples of (1) leach solution alone, (2) leach solution with 2 pct ammonium chloride, and (3) leach solution with 2 pct ammonium chloride and 1 pct clay stabilizing cationic polymer. Each of the samples was mixed and sheared for 5 min in a blender prior to the measurement of zeta potentials of the suspended fire clay particles.
Clays exposed to leach solution: 1—no additives; 2—2 pct NH4Cl; 3—2 pct NH4Cl and 1 pct clay stabilizer.
Test results shown in table 3 indicate the zeta potentials of the fine clay particles treated with the cationic polymer are more electropositive than are the zeta potentials of particles treated with the simple ions of the leach solution or the leach solution with ammonium chloride. Used in proper concentrations, the cationic polymer is electropositive enough to neutralize the negative charge on fine clay particles and negate their natural dispersive forces. The means of the zeta potential values are well separated, and the standard deviations indicate that the differences are statistically significant.
Summary and Conclusions
Two uranium ores and one clayey copper ore were employed in simultaneous permeability and leaching tests to determine the effectiveness of an organic clay stabilizing polymer in preventing permeability loss. An alkaline sodium carbonate-bicarbonate lead solution of pH 8.5 and a sulfuric acid leach solution of pH 2 were used to leach the uranium ores. The acid solution was also used to leach the copper ore.
The permeability loss in the uranium ore sample greatest for the sodium carbonate-bicarbonate leach. Effluent analyses proved that sodium in the leach solution is exchanged for calcium in the clays. This results in increased clay swelling and dispersion and permeability loss. Treating the ore sample prior to leaching with a clay stabilizer decreases the attendant swelling and migration of the clays and reduces the permeability loss. The permeability of clay-stabilizer-treated ore was five times higher than that of untreated ore at the end of the 12-h leach test period.
Although the permeability loss in the uranium ore sample was less for the sulfuric acid leach than for the basic leach, permeability of treated ore was still twice that of untreated ore at the end of sulfuric acid leaching tests of 12-h and 14-day durations.
Acid leaching tests on a clayey copper ore indicated that clay-stabilizer pre-treatment could also reduce the permeability loss in acid leaching of porphyry copper ores. Permeability of treated copper ore was five times greater than that of untreated ore at the end of a 9-h leaching test.
Zeta potential measurements indicated that the zeta potentials of finely divided particles of sodium montmorillonite clay treated with clay-stabilizing polymer were more electropositive than untreated particles. This suggests that the negative charge on the fine clay particles was more than neutralized by the clay stabilizer, thereby negating the resultant dispersive forces and causing the clays to be flocculated and nondispersed. A clay-swelling test also confirmed that the clay stabilizer reduced swelling in a sodium montmorillonite clay.
On the basis of the laboratory results of this investigation, it is concluded that pretreatment clayey ores with a clay stabilizer will reduce clay swelling and dispersion and thus reduce permeability losses. Some retardation in metal extraction was observed when a pretreated uranium ore was leached. This possible disadvantage in using clay stabilizers could be minimized if the stabilizer were used to treat only the small volume of ore within the zone of high fluid velocity around the injection well. In this case, the reduction in metal would be minimized, while the savings accrued by reducing well maintenance costs and ensuring adequate movement of leach solution into the ore formation would be significant. Field testing of clay stabilizers is needed to prove this hypothesis.
 |
 |
 |
 |
 |
