Table of Contents
Several interesting experiments were made in the sampling of the silver-lead bullion obtained from the water- jacket smelting-furnace. The smelting-plant is divided into two portions, known locally as the north furnaces and the south furnaces. The former group comprises nine and the latter six 80-ton water-jacket furnaces, besides which the south group has one small furnace used for smelting dross and concentrating the matte which is obtained in the larger furnaces, etc.
At furnaces, the lead is tapped into lead-kettles and allowed to stand for a short time, then skimmed and ladled into moulds fixed on a stand alongside the furnace. When the lead has set, the bars are taken out of the moulds and stacked, as shown in Fig. 1. At the south furnaces, the bullion is tapped from the furnace into a pot, and, while still molten, is wheeled over and tipped into a liquation-furnace, where it is purified. When enough bullion has accumulated in this furnace to make one lot (150 bars), the lead-tap is opened, and the lead is allowed to run into moulds placed on a revolving tray, which holds 150 moulds. These are generally filled at one tapping, and hold about 5 tons of bullion, or about 80 pounds to each bar.
The writer is indebted to Mr. W. J. Koehler, chief metallurgist, and his assistant, Mr. A. E. Savage, for the different methods of sampling the bullion. The samples were all carefully taken under their supervision and sent to the assay-office. The object of these experiments was to ascertain the relative accuracy of the methods then in use at the furnaces. The following results will be interesting to readers connected with metallurgical works.
Bars from the Liquation Furnace
A lot of 150 bars, taken from the liquation-furnace, was sampled and assayed as follows:
(A) Usual method of sampling, i.e., one dip-sample is taken at the beginning, one in the middle and one at the end of the run. Equal weights of these dips are taken, hammered into sheets about 1/8-inch thick, and then cut into small pieces. These are thoroughly mixed, and 0.25 assay ton is weighed out for assay. All assays are made at least in triplicate. The assay result was 469.4 ounces per ton. The three dips were assayed separately, with the following results:

(B) As the bullion was running from the furnace into the moulds, a dip-sample was taken at every tenth bar, making fifteen samples altogether. These were hammered into thin sheets, and assays were made from each, with the following results :

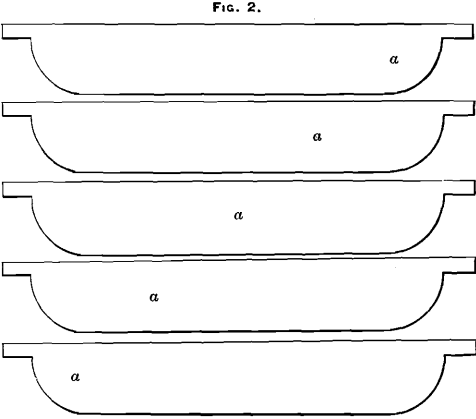
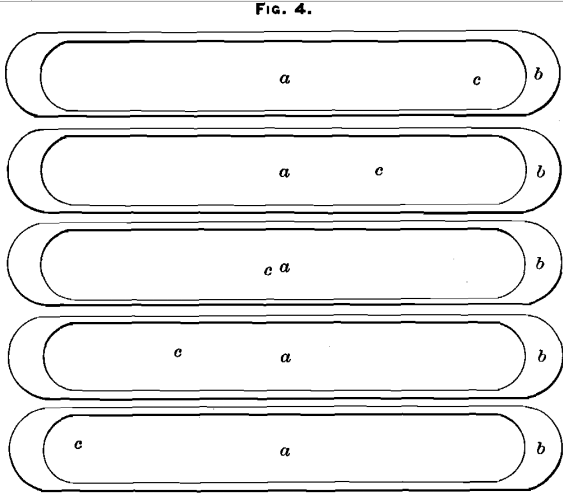
(C) Sample taken from the lugs, five bars being chipped on the top and five on the bottom, alternately (at points b, Figs. 3 and 4).
Assay-result, 482.7 ounces per ton.
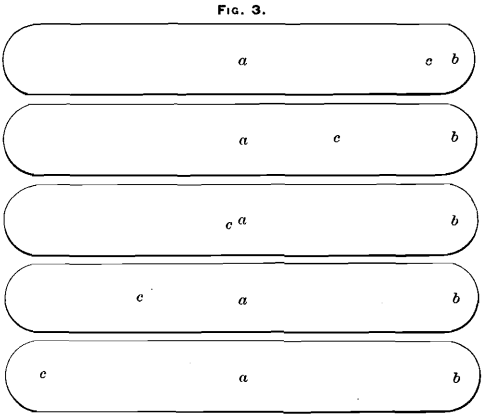
(D) Sample taken from the center of the bottom of the bars (points a, Fig. 4).
Assay-result, 471.1 ounces per ton.
(E) Sample taken from the center of the top of the bars (points a, Fig. 3).
Assay-result, 439.1 ounces per ton.
(F) Sample taken diagonally across the top of the bars as they were stacked (points c, Fig. 3).
Assay-result, 469.1 ounces per ton.
(G) Sample taken diagonally across the bottom of the bars (points c, Fig. 4).
Assay-result, 466.5 ounces per ton.
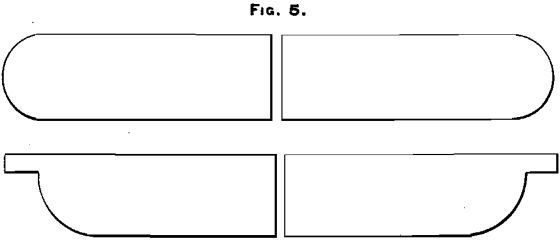
(H) Sample taken by sawing each bar through transversely (see Fig. 5). The saw-dust obtained amounted to about 2 cwt. This was carefully mixed, then quartered, and each quarter was separately cut down by another quartering, until four samples remained, of about 3 pounds each. These were each washed with benzine, to remove any oil or greasy matter derived from the saw. In this washing operation the samples lost 0.25 per cent, in weight. The assay-results were:

Comparing the different results, we find that the average samples taken agree very closely. They are for

This proves undoubtedly that the method in use, of taking the dip-samples from the bullion passing through the liquation- furnace, is perfectly correct. When this furnace was first started, the first four lots of bullion tapped from it were sampled by both methods A and B before adopting the former. In every case the results were the same. Samples F and G show that in this lot of bullion there was very little difference in value between the top and bottom of the bars; but, contrary to expectation, the top-sample goes slightly higher. Only in cases where the bullion is sampled by chips is this point important. The greatest variation occurs in samples C and E, and there seems to be nothing to indicate the reason. The top of the bar is invariably poor, and the lugs and sides are the richest.
Bars from the Lead-Kettles
A lot of 150 bars, moulded directly from the lead-kettles at the north furnaces, was sampled as follows:
(I) After a kettleful of lead had been tapped, the bullion was carefully skimmed, and a dip-sample was taken near the beginning and towards the end of the ladling. Each lot of bars thus tapped, varying from 11 to 16 bars, was weighed separately, until the whole lot of 150 bars had been made up. In the assay-laboratory, aliquot parts of the dips (corresponding to the weights of the different lots of bars) were weighed out and melted into one general sample.
Assay-result, 408.7 ounces per ton.
(J) Samples taken by the old method, with the bars stacked (as in Fig. 1), giving to every ten bars six bottom- and four top-samples. The samples were taken diagonally across, as shown (points a, Fig. 1).
Assay-result, 403.5 ounces per ton.
(K) Sample taken from five bars center of top and five bars center of bottom, alternately (points a, Figs. 3 and 4).
Assay-result, 403.2 ounces per ton.
(L) Sample taken from the lugs, five top and five bottom, alternately (points b, Figs. 3 and 4).
Assay-result, 407.5 ounces per ton.
(M) Sample taken diagonally across the top of the bars (points c, Fig. 3).
Assay-result, 396 ounces per ton.
(N) Sample taken diagonally across the bottom of the bars (points c, Fig. 4).
Assay-result, 408.9 ounces per ton.
The average of E and F is 402.5 ounces per ton, and represents the result obtained by the present method of sampling.
(O) Sample taken by sawing through the bars (see Fig. 5) and treating the saw-dust obtained like the corresponding sample (H) taken of bullion from the liquation-furnace.
The assay-result was:

It will be seen that there is a considerable difference (in this case, 12.9 ounces per ton) between (M), taken from top, and (N), taken from the bottom of the bars; so that, in sampling six bars at bottom and four bars at top (as would be the case in taking samples from bars stacked, as shown in Fig. 1), the
advantage would be in favor of the seller. In this particular case, however, it amounts to only 1 ounce per ton.
What is most noticeable is the high and concordant results obtained by the two only really reliable samples, namely, (I) the dip- and (O) the saw-sample. They are for (I) 408.7 and for (O) 409.45 ounces per ton.
The results of the above experiments point to the fact that samples taken with the gouge (that is, chip-samples) from the top and bottom of the bars are inaccurate, and always too low. The best remedy in this case would be to run all the bullion obtained from the north furnaces through a liquation-furnace, thereby providing a cleaner bullion, and also making it easy to obtain a dip-sample while the lead is being discharged into the moulds, as was the case in the experiments described in Section I., which prove the dip-sample to be, under such circumstances, perfectly accurate.
Gouge & Chip Sampling
Eight lots of bullion were sampled by the old method, the bars being stacked as in Fig. 1, and 6 samples being taken from the bottom and 4 from the top. They were then re-sampled by taking a chip out of the top and bottom of each bar.
The assay-results were as follows:
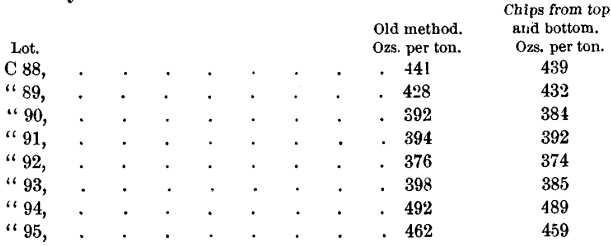
In all these lots except No. 89 the new method gives lower results than the old. Except as to Nos. 90 and 93, the difference amounts to from 2 to 3 ounces. This, in consideration of the further fact that both the above methods give results lower than either of the only accurate samples, namely, the dip- and the saw-samples, shows a great loss to the producer, and a corresponding gain to the refiner, by the adoption of chip-samples.
Distribution of Silver in the Bar
It will be noticed that, for both lots of bullion described in Sections I. and II., the results obtained by the dip- and saw-samples are higher than those obtained by the gouge- or chip-sample, in sampling the bars (at points c, in Figs. 3 and 4 combined). This can only be explained on the assumption that the silver contents of the bullion vary in different parts of the bar, and that the richer parts escape sampling by the gouge- or chip-method, but are fairly represented in both the dip- and saw-samples. In order to collect data on this point, the following samples were taken:
Lot I., from the liquation-furnace, was sampled by taking a gouge from the very center of the bar along the sawn surface.
Assay-result, 462 ounces per ton.
Lot II., north furnace bullion, was similarly sampled.
Assay-result, 400.3 ounces per ton.
In both these cases the results were lower than those of the dip- and saw-samples, which were 469.4 and 409.45 ounces respectively. The richer part of the bar, therefore, is not touched by the gouge-sample, but is represented in a dip- or saw-sample.
A section three-quarters of an inch thick was cut out of the center of two bars and laid off into nine portions, as shown in Figs. 6 and 7. Each portion was assayed separately, and the results will be seen in the two figures. Fig. 6 represents

a bar from the liquation-furnace. In this case the results are very irregular; but as this liquation-bullion is all sampled by the dip-method, this irregularity is not commercially important. Fig. 7 represents a bar from the north furnaces. It will be seen that the outside portions of the bar, numbered 1, 4, 7, 3, 6 and 9, are by far the richest. In a gouge- or chip-sample practically none of this rich material is taken. Some of the bullion in parts 1 and 3 is obtained, but only from the very top of the bar, which, as has already been shown, is poorer than the average of the bar (see M, Section II.); but in a saw- or dip-sample a fair proportion of all these parts is obtained (see Fig. 5).
A bar was taken from the liquation-furnace and sawn through transversely in three places; then each piece was sawn longitudinally at right angles to the upper face (see Fig. 8). The assay-results corresponding to the different saw-cuts are
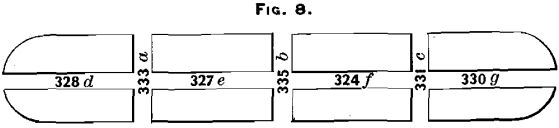
indicated on the figure. The assays were made on the saw-dust obtained from each cut. The dip-assay of this bar gave 334 ounces per ton.
Another bar was taken, and sawn as shown in Fig. 9. Two cuts were made longitudinally across the bar parallel to its upper face, and the three strips thus made were sawn through transversely, as shown in the figure. The results of assays are marked on the figure in the different saw-cuts. Dip-assay of bar, 336 ounces.
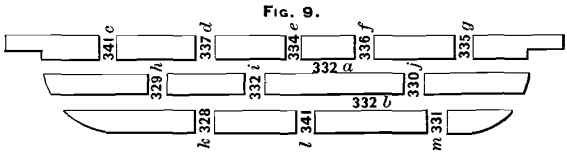
No definite data can be obtained from these results as to the composition of the interior of the bar in various parts, but they are interesting as showing that the silver-contents are very unevenly distributed through it.
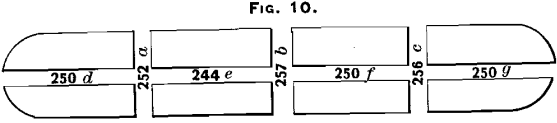
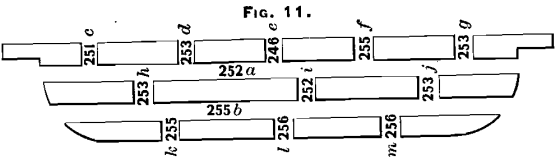
Two bars were taken from the north furnaces and treated as explained in the preceding paragraph. The results of assays are marked in the different saw-cuts shown in Figs. 10 and 11. In Fig. 10 the middle cross-section shows a higher result than any of the others, while in Fig. 11 the three middle cuts exhibit about the average of the other cuts, containing the lowest assay at e, the highest at l, and an average at i. It will be noticed in Fig. 11 that the samples contained more of the outside of the bar, which lies against the mould, and that, with the exception of the top-center saw-cut, the samples agree much more
closely than is the case in Fig. 10.
Samples were taken from the outside (i.e., that portion of the bar lying against the mould) of the bars from the liquation-furnace. Nos. 1, 2, 3, 4, 5, 6 and 7 were taken from the sides of the bar shown in Fig. 8, and Nos. 8, 9, 10, 11, 12, 13 and 14 were taken from the sides of the bar shown in Fig. 9. Samples 4, 5, 6 and 7 were taken just under the upper edge of the top of the bar, while the rest were taken about two-thirds down the side of the bar. The following are the assay-results:
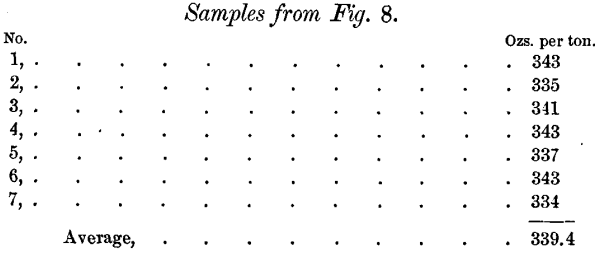
The dip-assay of this bar was 334 ounces per ton, showing that the outside, in contact with the mould, is the richest part of the bullion. None of this is obtained in the gouge- or chip-sample.
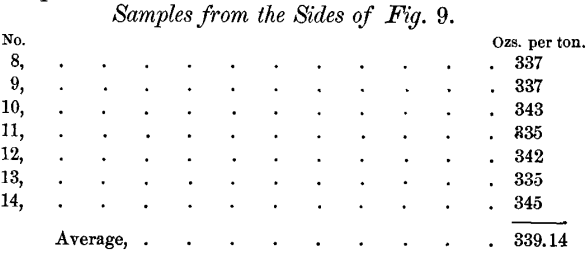
The dip-assay of this bar was 336 ounces per ton. These results confirm the statement made above.
The two bars from the north furnaces, shown in Figs. 10 and 11, were sampled from the outside. Only four samples were taken. The assay-results were:

On comparing these assays with the others shown on Figs. 10 and 11, it will be seen that in all cases they are as high as, or higher than, the results obtained in any other part of the bar.
In order to gain further evidence as to the richness of the outside of the bars, samples were taken from the bullion, experiments upon which are reported in Sections I. and II. of this paper. The lots were sampled by taking a chip out of the side of the bar, the chips being distributed equally along the sides from top to bottom; i.e., every five bars were sampled as shown by points a, Fig. 2.
Lot I. assayed 475 ounces per ton, against 469.4 ounces by dip- and 470 ounces by saw-sample.
Lot II. assayed 412 ounces per ton, against 408.7 ounces by dip- and 409.4 ounces by saw-sample.
These series of tests seem to establish the fact that there is no regularity in the distribution of the silver inside the bar, but that that part of the bar which lies directly against the sides of the mould is the richest, as already observed. Nothing from this part of the bar is obtained by the gouge-method of sampling the bars on top and bottom, in use up to the time when these experiments were made; but this portion is represented in a dip- or saw-sample. Hence, these two classes of samples give higher and far more accurate results than chip- samples.
Several additional tests were made to prove further the accuracy of the dip- and saw-samples, and in all cases the two agreed to within half an ounce per ton. Other tests, taken from the sides and lugs of the bars, always assayed higher than the correct value of the bar.
Loss of Lead by Volatilization
The question of the loss of lead by volatilization in pouring or melting, and the consequent enrichment of the sample in silver, has been thoroughly studied by the writer. Samples of bullion from the liquation-furnace were weighed before and after melting, with the following results:
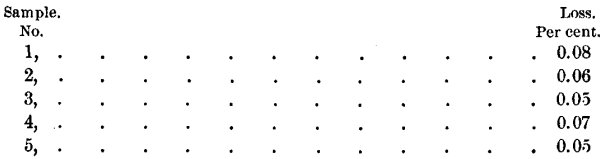
This loss, however, was not due to volatilization, but was caused by small particles of lead adhering to the sides of the crucible and sticking to the rod used for stirring. Three samples of bullion from the same lot were tried, as follows: 1500 grains were carefully weighed, placed in a red-hot crucible, and melted without stirring, a quick circular movement being given to the crucible instead. The lead was then poured into a small mould, and when cold was reweighed. The difference in weight was nil in two of the three cases, and the third gave a loss of half a grain, which would be 0.03 per cent. These tests prove conclusively that with care there need be no loss in melting soft bullion. As no oil is used in sampling by the gouge, treatment with benzine was omitted, except in the case of the saw-samples, which were greasy, owing to the saw having been well lubricated during the process. These were, therefore, all treated with benzine before melting down, and in this process showed a loss of 0.25 per cent., due to oil and greasy matter.
Of the bullion from the north furnaces, which is tapped direct from the furnace into a lead-kettle, then skimmed and ladled into moulds, two samples were melted, as above. The loss was 0.166 per cent., caused principally by a little drossy matter, which forms and adheres to the crucible; also a few globules of lead, which sometimes stick to the crucible. In the case of the bullion from the liquation-furnace no dross forms, the lead having been considerably purified in passing through this furnace. The writer is of opinion that there should be no loss in melting soft bullion if care is exercised and the bullion is not allowed to remain in the crucible after it becomes molten. There is no loss by volatilization when bullion is poured as soon as melted.
In connection with lead-bullion assays, the writer always melts in a red-hot crucible, which is preferable to the ordinary method of putting the crucible and lead into the furnace to melt. It has the advantage of quickness and greater command over the work; and, by several tests which he has made in melting the ordinary bullion-samples, the writer has found it an admirable way of melting lead-bullion. The samples seem to be more homogeneous when melted without placing in a hot fire; the checks have come out very close; and where, previously, five or six checks had to be made, only one or two were found necessary after adopting the hot-crucible method. Besides, the danger of loss is considerably reduced. The crucibles are kept hot in a furnace alongside the operator.
There is a slight loss in hammering the samples, due to small pieces of lead breaking off from the edges; but this makes no difference in the result of the assay, as the pieces are of similar composition to the sample assayed.
These tests prove conclusively that, with careful work, no lead is volatilized, and consequently no enrichment in silver contents of bullion takes place, in melting the bullion-samples.
The loss in cupellation is not great with this bullion. Experiment showed a loss of 0.015 per cent., or, on 400-ounce bullion, 0.06 ounce per ton. The test was made by making a sample of similar composition to the bullion-sample and cupelling it with the bullion-sample in the same muffle- furnace. The loss in this instance was not of much importance; but, as a general rule, the operator needs to be very careful in cupelling bullion-samples, as the loss may otherwise be great.
Summary
Samples taken by chipping out of the top and bottom of each bar almost always give a result lower than the actual value of the bullion. This is due to the fact that that part of the bullion lying against the sides of the mould is the richest in silver, and that none of it is obtained in the chip-sample. If chip-samples are to be taken, it would be fairer to take chips from the top, bottom and side of each bar, these to be taken diagonally across the five bars when stacking. The tests reported in this paper prove that the only really accurate samples are the dip-sample and the saw-sample.