Table of Contents
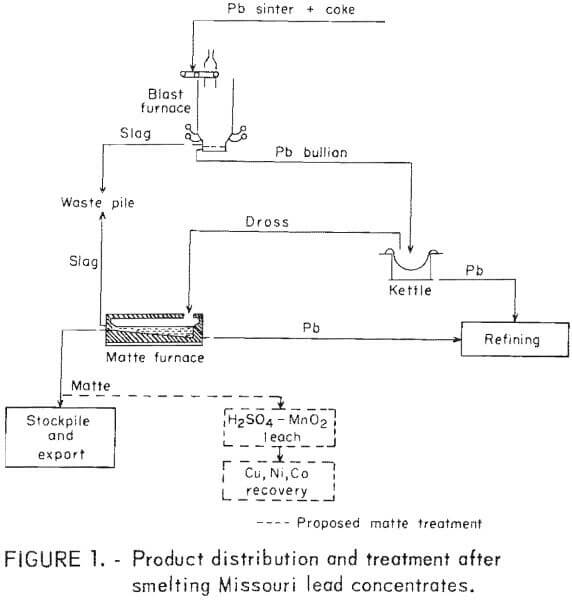
It has long been recognized that the 9 million tons of lead ore mined annually from Missouri’s Lead Belt (83 pct of the U.S. domestic production) contain significant amounts of nickel and cobalt. Yet, neither of these important commodities is being recovered by the current processing depicted in figure 1. The pyrometallurgical treatment of primary dross after smelting Missouri Lead Belt concentrates produces 6,000 tons/yr of mattes containing 2,300 tons of copper, 500,000 pounds of nickel, and 90,000 pounds of cobalt. Missouri processors are currently stockpiling and exporting the mattes under severe financial penalties because of arsenic and nickel contents that are deleterious to environmentally acceptable copper recovery techniques.
As part of its goal to maximize the recovery of minerals and metals from primary and secondary resources, the Bureau of Mines is performing research directed toward the recovery of copper, nickel, and cobalt from lead smelter mattes. The objective of this work is to demonstrate the recovery of these metals using readily available reagents and techniques which could be transferable to existing milling and smelting operations. The successful leaching of lead smelter mattes as described in this report has led to subsequent investigations that indicate an environmentally sound hydrometallurgy scheme for processing Missouri lead smelter mattes.
Materials and Equipment
Lead Smelter Mattes
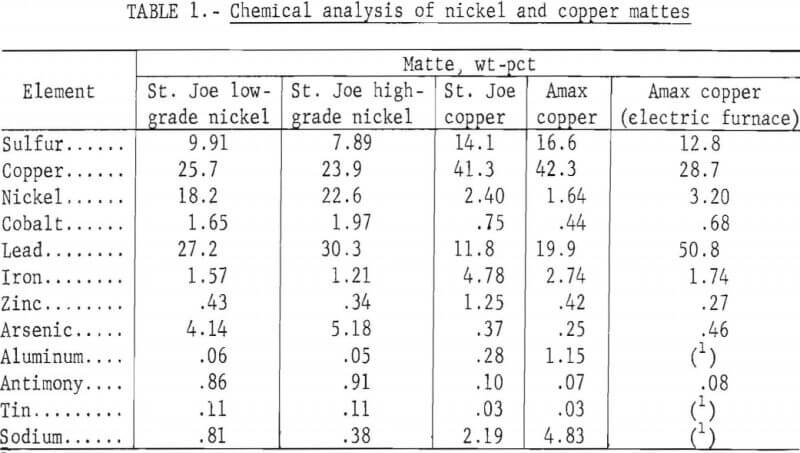
 Mattes were obtained from the St. Joseph Mineral Corp. lead smelter at Herculaneum, Mo. and the Amax Lead Co. of Missouri lead smelter at Boss, Mo. The St. Joe lead smelter produces three grades of matte, a low- and a high-grade nickel matte and a copper matte, which makes up the greatest portion of the mattes. The Amax lead smelter produced previously a copper matte and a speiss (high nickel and arsenic), but recently has started producing a copper matte with more lead, nickel, and arsenic, without the production of speiss, by using an electric smelting furnace. Chemical analyses of the mattes produced at both lead smelters are shown in table 1. Preliminary X-ray diffraction analyses of the nickel and copper mattes from the St. Joe smelter are listed in table 2. The particle sizes of the nickel mattes as received were 91 pct plus 65 mesh. The as-received copper mattes were 48 pct plus 65 mesh. These mattes were curshed in a horizontal crushing rolls and then ground in a small laboratory ball mill to an average particle size of about 260 µm for use in the leaching tests.
Mattes were obtained from the St. Joseph Mineral Corp. lead smelter at Herculaneum, Mo. and the Amax Lead Co. of Missouri lead smelter at Boss, Mo. The St. Joe lead smelter produces three grades of matte, a low- and a high-grade nickel matte and a copper matte, which makes up the greatest portion of the mattes. The Amax lead smelter produced previously a copper matte and a speiss (high nickel and arsenic), but recently has started producing a copper matte with more lead, nickel, and arsenic, without the production of speiss, by using an electric smelting furnace. Chemical analyses of the mattes produced at both lead smelters are shown in table 1. Preliminary X-ray diffraction analyses of the nickel and copper mattes from the St. Joe smelter are listed in table 2. The particle sizes of the nickel mattes as received were 91 pct plus 65 mesh. The as-received copper mattes were 48 pct plus 65 mesh. These mattes were curshed in a horizontal crushing rolls and then ground in a small laboratory ball mill to an average particle size of about 260 µm for use in the leaching tests.
Reagents
Sulfuric acid used in the matte leaching experiments was either a reagent- grade (95 to 98 pct H2SO4) or black acid. The black acid, obtained from a Missouri lead smelter, was produced by the Monsanto contact acid process in which the SO2-laden gas from the sintering machine was purified, cooled, and then contacted with a vanadium pentoxide catalyst to accelerate the reaction between SO2 and ½ O2 to form SO3. The SO3 then was absorbed in sulfuric acid that contained 96.4 pct sulfuric acid, and several trace elements such as carbon, calcium, and iron.
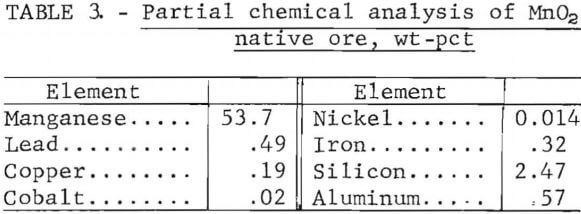
Reagent-grade manganese dioxide (99.6 pct MnO2) was used as an oxidizing agent in most of the leaching tests. This reagent had an average particle size of 75 µm and was used as-received. In addition to the reagent-grade manganese dioxide, a native manganese dioxide ore was used in several tests. The chemical analysis of the manganese dioxide native ore is shown in table 3.
The native ore was used as-received at an average particle size of 27 µm,
Procedure
The Amax copper and St. Joe nickel mattes were leached in a 1,000-ml, three-neck, round-bottom Pyrex glass flask. The heat to the reaction flask was supplied with a heating mantle controlled by a powerstat. The temperature was monitored with a digital temperature indicator capable of ±0.1° C. The temperature indicator was fitted with a type-J thermocouple in a glass thermocouple well. To minimize liquid loss, the steam outlet of the reaction flask was connected to a West condenser. Agitation of the leach slurry was performed with a variable-speed mixer motor, a glass bearing and shaft, and a 2-inch, single-blade Teflon paddle (fig.2).
The leach slurries were filtered through No. 54 Whatman filter paper (11 cm in diameter) into a 1,000-ml filter flask that was inder 38 cm Hg vacuum.
Most leaching tests were performed to obtain a material balance for comparison of operating parameters. Generally, tests were conducted over 1- to 5-hr periods; operating conditions of temperature (±0.1° C), charge weights (±0.01 g), and volumes (±2 ml) were maintained. The filtration rate (+1 sec) was recorded when the hot slurry was vacuum filtered. The final filtrate volume (+2 ml) and pH (+0.1 unit) were recorded at room temperature.
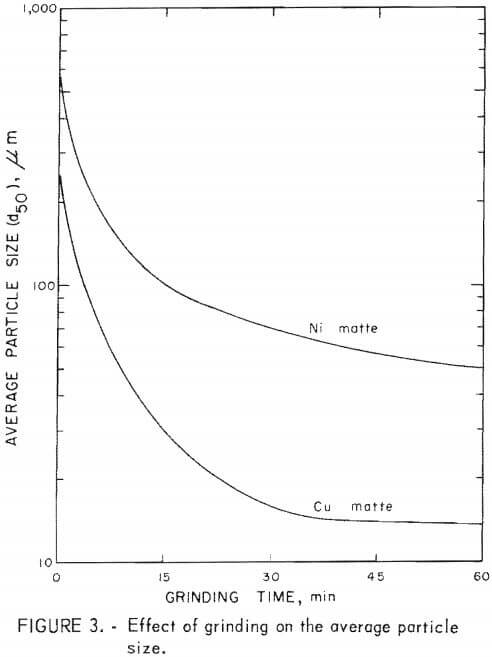
Initial sample perparation consisted of predrying and grinding. The matte samples were air-dried at 105° C overnight. Average particle size (d50) was determined after grinding the matte in a 21.6-cm laboratory steel ball mill for specific times. The relationship between average particle size (d50) and grinding time is shown in figure 3. The Amax copper matte was ground and sized wet, then dewatered and dried before use in the leaching runs. The St. Joe nickel matte was ground dry, and a sample was sized wet. Dry material was used in the leaching runs.
The leaching reactor was loaded by premixing the acid with water, then pouring it into the reactor, and heating to within 15° C of the operating temperature. Due to the exothermic nature of the reaction, maganese dioxide and matte were added slowly to the sulfuric acid and water mixture. The agitator speed, water flow to the condenser, and reactor temperature were measured and recorded throughout the tests.
All power and water was shutoff at the end of each run, and the contents of the reactor were filtered hot. The residue was washed with distilled water, which then was added to the final filtrate. The final filtrate volume and pH were measured at room temperature, and an aliquot was analyzed.
 |
 |
All analyses were performed on an atomic absorption spectrophotometer using the flame method. Liquid samples were diluted, while solid samples were dissolved in nitric acid (HNO3), and then diluted of an appropriate volume. Metal concentrations were reported as grams per liter for liquids and percent for solids.
Results
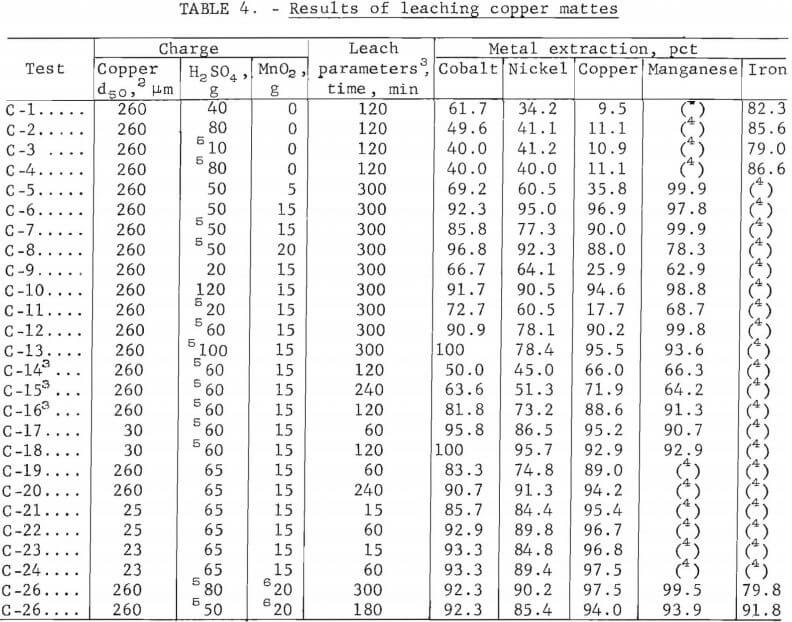
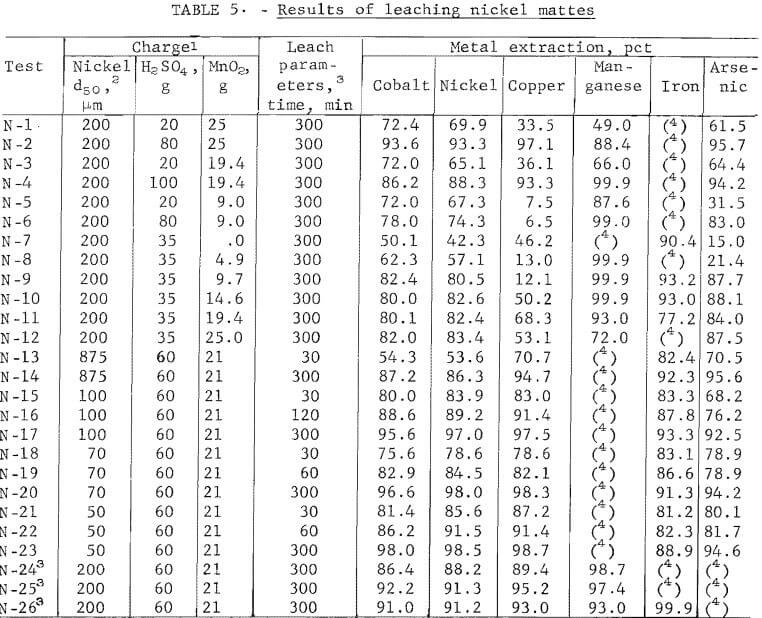
The results of 50 pct of the experimental leaching tests on copper and nickel mattes are presented in tables 4-5. Data are presented for selected individual runs that show specific effects of charge and operating parameters. The percentage extracted was calculated from a mass balance for each run, as indicated from the analyses of the residues and leach liquors. Because of the similarity of the data obtained from the copper and nickel mattes, only the black acid and native ore manganese dioxide leach results from copper matte are shown. Leach liquors resulting from an oxidative leach of copper matte with sulfuric acid and manganese dioxide contained (in grams per liter) 0.5 cobalt, 1.2 nickel, 33 copper, 35 manganese, 2.3 iron, and 0.29 arsenic. The nickel matte leach liquors contained (in grams per liter) 2.5 cobalt, 20 nickel, 28 copper, 50 manganese, 1.7 iron, and 7 arsenic.
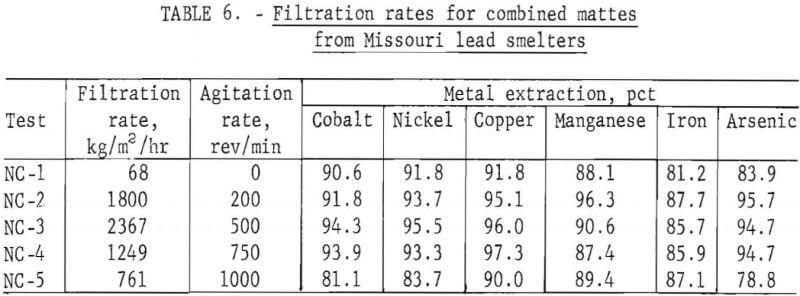
The effect of agitation on the filtration rate and metal recoveries is shown on table 6. Tests were run with various combinations of mattes in an attempt to determine any problems associated with leaching lead smelter processing production ratios of copper and nickel mattes. The rate of filtration and extraction results were obtained during the testing of equal ratio of copper and nickel mattes . The recoveries were calculated from a mass balance of each run and in this paper are equivalent to the present solubilized.
Discussion
Effects of Reagents
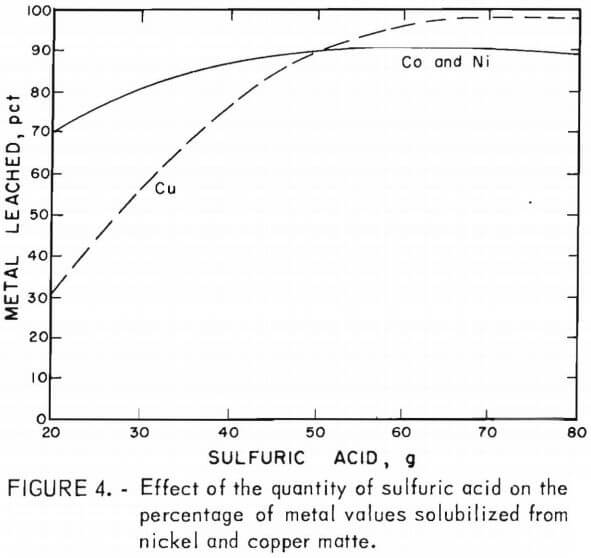 Sulfuric acid leaching of lead smelter mattes resulted in the recovery of about 45 pct of the nickel and cobalt and 10 to 45 pct of the copper. The addition of manganese dioxide increased the cobalt and nickel recovery from 45 pct to greater than 90 pct, and increased the copper from 10 to 45 pct to nearly 100 pct recovery. The cobalt and nickel extractions were found to be less dependent on the concentration of sulfuric acid and manganese dioxide than the copper extraction. This indicates that the nickel and cobalt are possibly present in the mattes as oxides and the copper as a sulfide. The results of the tests using varying amounts of acid with constant amounts of manganese dioxide and copper or nickel matte are shown in figure 4. Copper matte tests were run with 15 g of manganese dioxide and varying amounts of sulfuric acid. Nickel matte was leached with either 19.4 or 25 g of manganese dioxide and varying amounts of sulfuric acid; equivalent leaching results were obtained.
Sulfuric acid leaching of lead smelter mattes resulted in the recovery of about 45 pct of the nickel and cobalt and 10 to 45 pct of the copper. The addition of manganese dioxide increased the cobalt and nickel recovery from 45 pct to greater than 90 pct, and increased the copper from 10 to 45 pct to nearly 100 pct recovery. The cobalt and nickel extractions were found to be less dependent on the concentration of sulfuric acid and manganese dioxide than the copper extraction. This indicates that the nickel and cobalt are possibly present in the mattes as oxides and the copper as a sulfide. The results of the tests using varying amounts of acid with constant amounts of manganese dioxide and copper or nickel matte are shown in figure 4. Copper matte tests were run with 15 g of manganese dioxide and varying amounts of sulfuric acid. Nickel matte was leached with either 19.4 or 25 g of manganese dioxide and varying amounts of sulfuric acid; equivalent leaching results were obtained.
All of these leaching tests included 25 g of copper or nickel matte, a 5-hr reaction time, a temperature of 90° to 95° C, and a total solvent volume of 200 ml. Some of the data shown in figure 4 are from tests C-9 – C-10 6 in table 4 and N-1 – N-4 in table 5. Lines shown in the graph depict the percentage of metals leached and represent averages of the cobalt, nickel, and/or copper values solubilized in a given test series. These results show that cobalt and nickel extraction from the mattes was not strongly dependent on the sulfuric acid concentration. Copper solubilization was greatly dependent on the amount of sulfuric acid. There was a fairly linear increase from 30 to 90 pct recovery of copper from the mattes by varying the amount of sulfuric acid from 20 to 50 g.
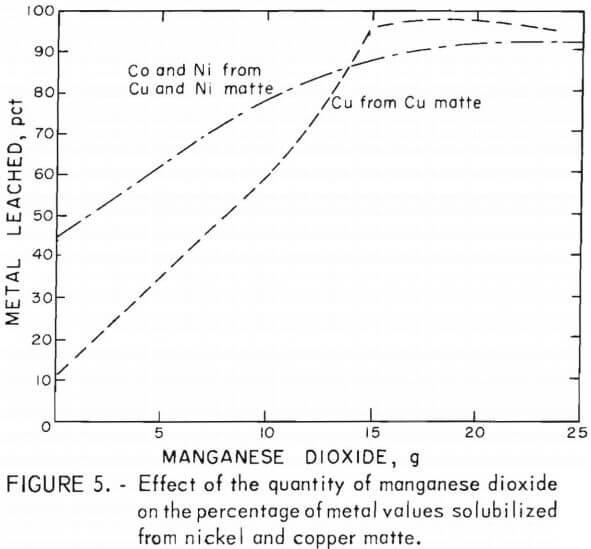
The results of varying the manganese dioxide additions at a constant sulfuric acid concentration when leaching the copper and nickel mattes are shown in figure 5. Some of the data represented in the graph are from tests C-5 – C-6 in table 4 and N-7 – N-12 in table 5. Leaching tests on 25-g batches of copper or nickel matte included varying amounts of manganese dioxide, a 5-hr reaction time, a temperature between 90° and 95° C, and 35 or 50 g of sulfuric acid in total solvent volumes of 100 or 200 ml for nickel and copper mattes, respectively. The results of these tests demonstrate the large effect manganese dioxide has on leaching metal values from lead smelter mattes. The graph in figure 5 shows that cobalt and nickel recovery was increased from 45 pct to greater than 90 pct by the addition of manganese dioxide to the sulfuric acid leach on both the copper and nickel mattes. A much larger effect was observed in the case of copper, particularly when leaching copper matte. The copper solubilized from copper matte was increased from about 10 pct to greater than 95 pct with the addition of manganese dioxide to the sulfuric acid leach. Testing on nickel mattes showed that adding 60 g of sulfuric acid in 200 ml of solvent with 21 g of manganese dioxide resulted in up to 98 pct copper extraction as shown in table 5, tests N-20 and N-23.
 Tests were run using black acid as a substitute for reagent-grade sulfuric acid because of its low cost and availability to lead smelters, and to determine any undesirable affects on leaching copper matte. The major differences between the two acids was the sulfuric acid concentration and the trace amounts of impurities such as iron, calcium, other metals, and a small quantity of organic material contained in the black acid. Black acid was 94 to 96 pct sulfuric acid and reagent-grade sulfuric acid was 95 to 98 pct sulfuric acid.
Tests were run using black acid as a substitute for reagent-grade sulfuric acid because of its low cost and availability to lead smelters, and to determine any undesirable affects on leaching copper matte. The major differences between the two acids was the sulfuric acid concentration and the trace amounts of impurities such as iron, calcium, other metals, and a small quantity of organic material contained in the black acid. Black acid was 94 to 96 pct sulfuric acid and reagent-grade sulfuric acid was 95 to 98 pct sulfuric acid.
Data obtained from tests with black acid or sulfuric acid are shown in table 4, tests C-2 and C-4. The data indicates that impurities in the black acid will slightly lower the extraction efficiency as shown in a comparison of tests C-2 and C-4. Black acid with reagent-grade manganese dioxide was used to leach copper matte. These tests were run on 25 g of copper matte, varying amounts of black acid, for 5 hr at 95° C in a total solvent volume of 200 ml. The data from these tests are given in table 4, tests C-7 – C-8 and C-11 – C-18. The effect of manganese dioxide was about the same in black acid as in reagent-grade sulfuric acid. About a 10-pct decrease in leaching efficiency was observed at each manganese dioxide level.
In an effort to decrease the cost of replacing the manganese dioxide that may possibly be lost in its recycle, native manganese dioxide ore was tested. The native ore that was used contained about 80 pct manganese dioxide. Several tests were completed to determine how native manganese dioxide ore and black acid would react with copper matte (25 g) using various amounts of native ore and black acid over a range of leaching times. Some of these tests are shown in table 4, tests C-25 – C-26. Manganese dioxide native ore (80 pct MnO2) additions ranged between 17 and 25 g, the black acid ranged between 40 and 80 g, and the time ranged from 1 to 5 hr. The temperature was 90° C and the total solvent volume was 200 ml. Greater than 90 pct of the cobalt, nickel, and copper were solubilized with as high as 99 pct of the manganese. The major differences between the use of reagent-grade manganese dioxide and native manganese dioxide ore were the increased amount of native manganese dioxide ore required and the time required for metal extraction. About 25 pct more native ore and a 10- to 20-pct longer leach time were required. The increased quantity of native ore required was due to its 20- to 25-pct lower manganese dioxide content. Another possible problem that could be expected is that of impurity buildup due to the contained impurities in the native manganese dioxide ore. The impurity buildup can be held to a minimum since only the manganese dioxide that was not recovered during recycle must be restored.
Effects of Operating Parameters
Particle Size
Copper matte was found to be quite friable. The average particle size was decreased from 260 to 30 µm in 15 min with a laboratory ball mill. Further grinding decreased the average particle size from 30 to 23 µm in 30 min.
Nickel matte was more difficult to grind than copper matte. A 15-min grind of nickel matte lowered the average particle size from 875 to 100 µm and further grinding for 45 min lowered the average particle size from 100 to 50 µm.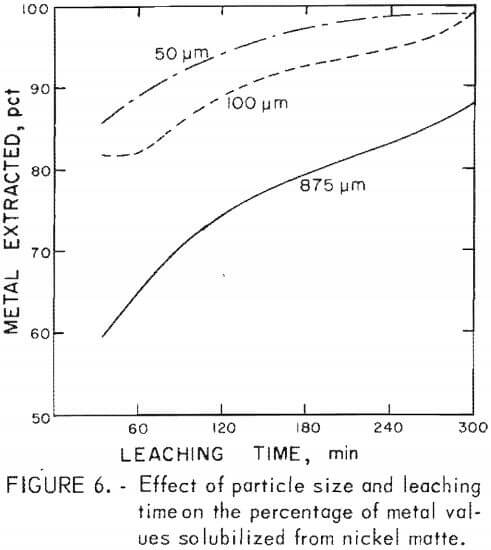
Matte particle size was found to have only a minor effect on metal extraction from copper matte, whereas particle size had an appreciable affect on metal recovery from nickel matte. Leaching time decreased with average particle size when treating copper and nickel mattes, but to a greater extent with nickel matte. The results of leaching nickel matte at various average particle sizes over a 5-hr time period are shown in figure 6. Lines shown in the graph depict the percentage of metals leached and represent averages of the cobalt, nickel, and copper solubilized in a given test series. These tests, including tests with copper matte, are given in table 4, tests C-17 – C-24 and in table 5, tests N-13 – N-23. The nickel matte tests were run with 25 g of matte, 21 g of manganese dioxide, and 60 g of sulfuric acid at 90° to 95° C over a period of 0 to 5 hr in a total solvent volume of 200 ml. The conditions for the copper matte tests were about the same except that 65 g of black acid and 15 g of manganese dioxide were used. The nickel matte leach improved significantly when the average particle size of the matte was lowered from 875 to 100 µm. Metal extraction increased by 3 to 30 pct at any point over the entire 5-hr leach. Greater than 80 pct of the cobalt, nickel, and copper was solubilized in less than 30 min when the average particle size of the nickel matte was 100 µm compared with less than 60-pct recovery of metals from nickel matte with an average particle size of 875 µm. Grinding the nickel matte to an average particle size of 50 µm and leaching resulted in an increase of metal extraction of only 5 pct over leaching the 100-µm material, Greater than 80 pct of the metal values were extracted from a copper matte in less than 60 min with an average particle size of 260 µm compared with greater than 90 pct recovery of metals with an average particle size of 30 µm. Leaching copper matte ground to 23 µm resulted in an increase in overall solubility by only a few percent, but the leaching time was reduced. About 90 pct metal extraction was realized in a 15-min teach with an average particle size of 23 µm compared with 80 pct metal extraction from an average particle size of 260 µm.
 Temperature
Temperature
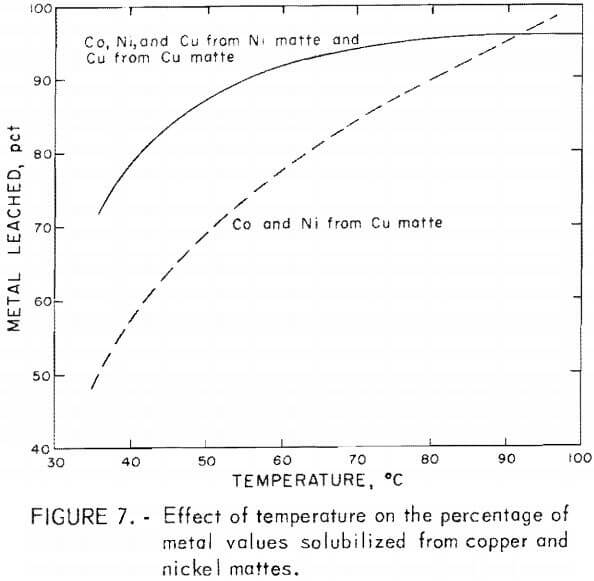
Temperature effects, between 35° to 100° C, were appreciable on leaching lead smelter mattes with the greatest effects occurring in the extraction of cobalt and nickel from copper matte. A much smaller effect was observed in the extraction of cobalt and nickel from nickel matte and copper from nickel and copper mattes. A review of the temperature effects is given in figure 7. The metals extracted are depicted as averages of the cobalt, nickel, and copper solubilized in a given test series. A portion of the information given in figure 7 was obtained from table 4, tests C-12 and C-14-C-16, and table 5, tests N-24-N-26. These results were acquired by leaching 25 g of nickel or copper matte with 60 g of sulfuric acid or black acid at a total volume of 200 ml for 5 hr using 15 and 21 g of manganese dioxide for leaching copper or nickel matte.
The percentage of cobalt and nickel solubilized from copper matte increased almost linearly from 35° to 100° C with an increase from 45 to 95 pct metal extracted. Recoveries of cobalt and nickel from nickel matte and copper from both copper and nickel mattes were increased from about 70 to 95 pct between 35° and 80° C with little increase in the percent of metal extracted above 80° C.
Agitation
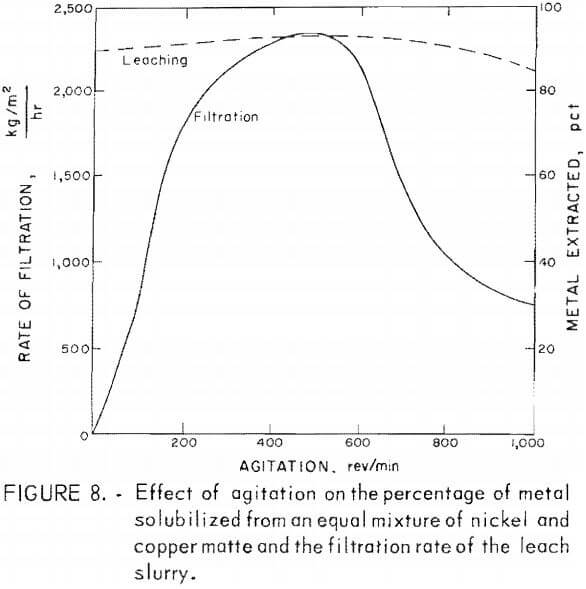 The effects of agitation rate on metal extraction from copper and nickel mattes were small. However, during the filtration of these slurries, the rate of filtration was found to be greatly dependent on the rate of agitation during leaching. The results of tests to determine agitation effects on extraction of metals and filtration rates are shown in figure 8. The data utilized in this figure are given in table 6, tests NC-1-NC-5. This information was obtained by leaching 25 g of an equal ratio of copper and nickel mattes, using 18 g of manganese dioxide, 50 g of sulfuric acid diluted to 200 ml. and reacted for 2 hr at 95° C, while being stirred at varying rates. The percentage of metal extracted during the leach was increased by only a few percent over the agitation range of 0 to 600 rev/min. The rate of filtration of these leach slurries increased steadily with leach agitation between 0 and 500 rev/min. The filtration rate increased from about 68 kg/m² hr with no agitation during leaching to near 2,367 kg/m² hr agitated at 500 rev/min. An increase in agitation rate from 500 to 1,000 rev/min during leaching resulted in a continuous decrease in the filtration rate.
The effects of agitation rate on metal extraction from copper and nickel mattes were small. However, during the filtration of these slurries, the rate of filtration was found to be greatly dependent on the rate of agitation during leaching. The results of tests to determine agitation effects on extraction of metals and filtration rates are shown in figure 8. The data utilized in this figure are given in table 6, tests NC-1-NC-5. This information was obtained by leaching 25 g of an equal ratio of copper and nickel mattes, using 18 g of manganese dioxide, 50 g of sulfuric acid diluted to 200 ml. and reacted for 2 hr at 95° C, while being stirred at varying rates. The percentage of metal extracted during the leach was increased by only a few percent over the agitation range of 0 to 600 rev/min. The rate of filtration of these leach slurries increased steadily with leach agitation between 0 and 500 rev/min. The filtration rate increased from about 68 kg/m² hr with no agitation during leaching to near 2,367 kg/m² hr agitated at 500 rev/min. An increase in agitation rate from 500 to 1,000 rev/min during leaching resulted in a continuous decrease in the filtration rate.
Cobalt, Nickel, and Copper Recovery
The sulfuric acid-manganese dioxide leaching of lead smelter mattes and subsequent processing to recover the contained cobalt, nickel, and copper are included in the proposed flow diagram shown in figure 9. The matte leaching produces a cobalt, nickel, and copper containing liquor that is contaminated with arsenic and iron. Iron-arsenic precipitation tests showed that these contaminants can be precipitated at a pH of about 4.0 with sodium hydroxide and lime, then the pH decreased to 3.0 with sulfuric acid to redissolve coprecipitated copper. As much as 88 to 92 pct of the arsenic, 98 pct of the iron, and 1 to 1.3 pct of the copper was precipitated from an equal copper-nickel matte leach liquor to yield a product containing 24 pct arsenic, 22 pct iron, and 3 to 5 pct copper.
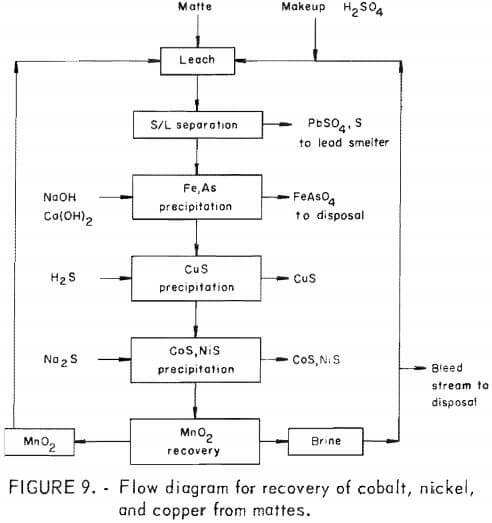 Sulfide precipitation is an effective way to recover the copper, cobalt, and nickel from the purified copper-nickel matte leach liquor. The addition of a near stoichiometric amount of hydrogen sulfide to the leach liquor at a pH of 0.7 to 2.8 and a temperature of 50° C resulted in 93 to 99 pct precipitation of copper, 0.38 to 0.47 pct of the cobalt, and 0.05 to 2.3 pct of the nickel. The resulting product contained 61 to 64 pct copper, and less than 0.01 pct cobalt and nickel. After removal of the precipitated copper sulfide from the leach liquor, further sulfiding of the liquor, at a pH near 3, with sodium sulfide resulted in the precipitation of 99 and 97 pct of the nickel and cobalt.
Sulfide precipitation is an effective way to recover the copper, cobalt, and nickel from the purified copper-nickel matte leach liquor. The addition of a near stoichiometric amount of hydrogen sulfide to the leach liquor at a pH of 0.7 to 2.8 and a temperature of 50° C resulted in 93 to 99 pct precipitation of copper, 0.38 to 0.47 pct of the cobalt, and 0.05 to 2.3 pct of the nickel. The resulting product contained 61 to 64 pct copper, and less than 0.01 pct cobalt and nickel. After removal of the precipitated copper sulfide from the leach liquor, further sulfiding of the liquor, at a pH near 3, with sodium sulfide resulted in the precipitation of 99 and 97 pct of the nickel and cobalt.
The precipitated product contained 33 to 45 pct nickel, 2 to 3 pct cobalt, and 0.3 to 1.6 pct manganese. The sulfide precipitations were monitored by a potentiometric method used commercially while precipitating copper from an iron-copper liquor. This procedure with some modification worked well while treating copper and nickel matte liquors.
The manganese remaining in the depleted copper, nickel, and cobalt leach liquor was precipitated as a carbonate with soda ash, and then converted to manganese or oxide by calcining at 350° C. The manganese oxide will be recycled to leaching. Leaching tests with recycled manganese oxide and sulfuric acid resulted in good extraction of metals from the matte.
Conclusions
Cobalt, nickel, and copper can be extracted from lead smelter mattes by leaching the ground material with manganese dioxide and sulfuric acid. Black acid, which is of low cost and available to lead smelters, can be used effectively in lieu of reagent-grade sulfuric acid. Oxidative leaching tests with sulfuric acid and recycled manganese dioxide resulted in good extraction of metal values from mattes. This technique appears feasible because of reasonable temperature and reaction time, the use of black acid, and the recyclability of manganese dioxide.
The Bureau of Mines, U.S. Department of the Interior, is investigating a hydrometallurgical procedure to conserve cobalt, nickel, and copper by recovering the metals from the matte byproducts of smelting Missouri lead ores. For want of adequate processing technology the mattes are now either unmarketable or marginally so. The procedure involves leaching with manganese dioxide and sulfuric acid to obtain copper, nickel, cobalt, lead, and manganese sulfates, and elemental sulfur as expressed by the following reaction:
MS + MnO2 + 2H2SO4 → MSO4 + MnSO4 + S° + 2H2O
where M = Cu, Ni, Co, and Pb.
The leach solutions, containing near theoretical amounts of sulfuric acid and manganese dioxide at 85° to 100° C, convert up to 98 pct of the cobalt, nickel, and copper in the mattes to sulfates in less than 2 hr. As they are formed, the cobalt, nickel, and copper sulfates are dissolved leaving a residue of lead sulfate, lead, sulfur, and gangue. The soluble metals are recovered from the leach solutions by caustic and sulfide precipitation, and the residue returned to the smelter for lead recovery. The manganese can be reclaimed from the spent leach liquor as manganese oxide and recycled to leaching.
