Table of Contents
As part of its mission to effect pollution abatement in mineral processing, we entered into a cooperative research project with 10 Florida phosphate mining companies. The objectives of the project were to seek means for disposing of phosphatic clay wastes and for reclaiming mined land.
Phosphatic slimes represent one of the principal waste products resulting from the beneficiation of Florida land-pebble phosphate ore. These waste-clay suspensions have colloidal characteristics that make them difficult to dewater. Consolidation of the waste clays by natural settling usually requires years to accomplish. The disposal method generally practiced is impoundment and long-term storage in ponds. Because of the enormous quantities produced, the impounded slimes represent serious potential pollution problems to the land and aquatic environment of the area. Boyle estimated that one phosphate plant alone produced 4 million tons of phosphatic waste clays per year, and Vasan estimated that nearly 2 billion tons of clays are stored behind the impoundment dams.
A detailed history of the Florida phosphate industry and the phosphatic clay waste problem is given by Blakey, and a comprehensive review and bibliography of research efforts to solve this problem has been published by the Bureau of Mines. In another study of phosphatic clay wastes, the physical, chemical, and mineralogical characteristics of slime samples from 15 operating plants were determined and compared. Information in this report indicated that the clay content of the wastes, particularly attapulgite and montmorillonite, significantly influenced settling rate and other behavior of the wastes.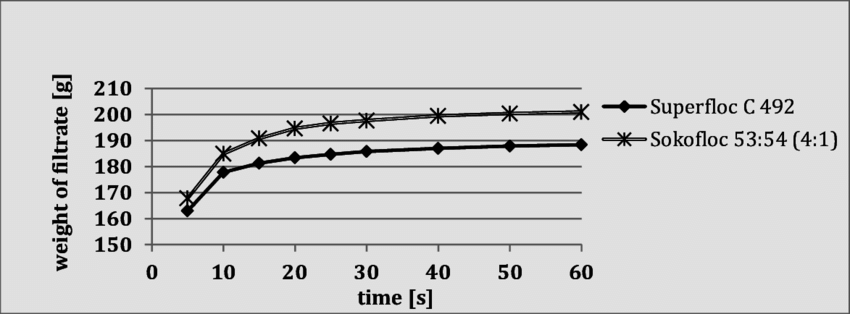
A recent report summarizes three methods of phosphatic clay-waste disposal that have been investigated in conjunction with the Florida Phosphatic Clays Research Project (FPCRP), a technical group representing the Florida phosphate mining companies and the Florida Phosphate Council. One of the methods described, called the sand spray process, was developed by Brewster Phosphates and is presently in full-scale use by that company. The technique has been tested at other plants under the direction of the FPCRP but has not proved successful in dewatering all slimes.
This report presents the results of Bureau research using a specialized flocculation method for dewatering phosphatic clay wastes. Application of the method in laboratory bench-scale tests and in larger scale continuous tests is herein described. All tonnages are metric.
Description of Samples Tested
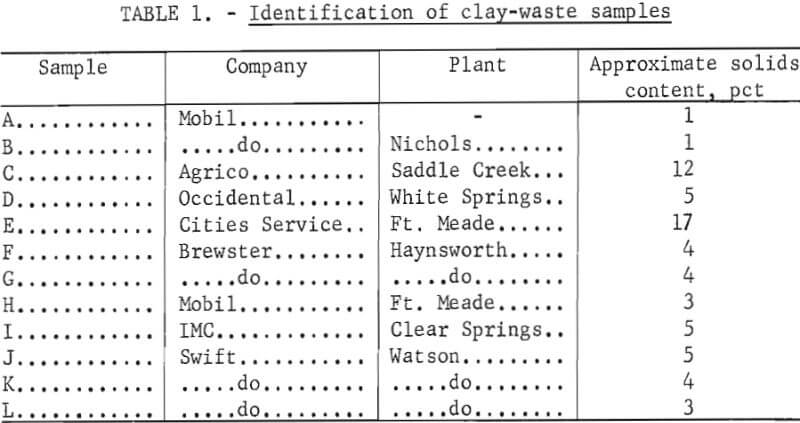
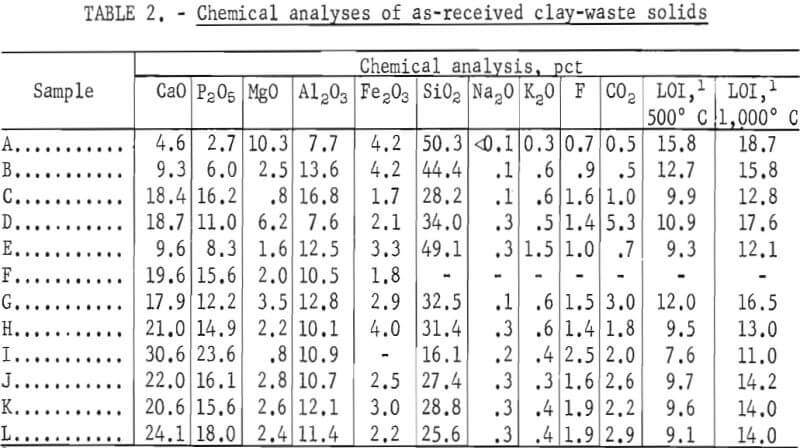
The slime samples used in this study were obtained from several of the cooperating mining companies. Identification of the samples and information about their sources are given in table 1. The samples of plant clay wastes were obtained as they were discharged from the phosphate-processing plants and were used in the solids concentrations as received. The solids from the suspensions were chemically analyzed, and characterized by X-ray diffraction and scanning electron microscope (SEM) techniques. Chemical analyses of the clay waste solids are presented in table 2.
Selected high-grade samples of attapulgite and montmorillonite, two of the major clay components of phosphatic clay wastes, also were obtained, and dilute suspensions of each were prepared for comparative flocculation studies. The slurries were made by mixing samples in distilled water, then screening and discarding the plus 325-mesh material. Analysis of the solids in the attapulgite suspension (sample A) by X-ray diffraction indicated that minor amounts of apatite, quartz, and montmorillonite were present. Solids in the high-grade montmorillonite suspension (sample B) contained minor amounts of apatite, quartz, wavellite, illite, and possibly a trace of attapulgite.
Initial Testing of Reagents for Flocculation
The investigation began as a basic study to evaluate the effect of various organic and inorganic compounds on the settling characteristics of the high-grade attapulgite and montmorillonite suspensions obtained from the phosphate land-pebble region. A wide variety of reagents, including nitrogen-bearing compounds, sulfur-bearing compounds, inorganic compounds, and numerous commercial reagents, were tested to determine their effects on the suspensions. The viscosity, conductivity, and pH of the mixtures were measured at 0.1-, 0.01-, and 0.001-molar reagent concentrations. To determine the effect of reagent and/or pH on settling, the mixtures of clays and reagents were adjusted to different pH levels by adding NaOH (sodium hydroxide) or HCl (hydrochloric acid), and then were centrifuged to simulate a 30-day settling period. Although additions of reagents generally affected the pH, viscosity, and conductivity of the slurries, most had no beneficial effect on settling.
One notable exception, however, was observed in the response of attapulgite suspension to HF (hydrofluoric acid). After a sample of attapulgite suspension was treated with HF, small flocs formed almost immediately, and settling began in less than 10 minutes. After 15 minutes, the solids had settled to 40 percent of the original volume. The settled material filtered easily. Suspensions of montmorillonite and phosphatic clay wastes from operating plants also responded to the HF treatment. Hydrofluosilicic acid and other fluoride compounds such as NH4F (ammonium fluoride) in acid medium caused the same type of settling behavior. Although the technique did not prove to be an economical method of treatment, the procedure has been patented by the Bureau.
Of all the organic flocculants evaluated in the study, one reagent demonstrated unusual behavior in flocculating and dewatering the attapulgite suspension. The reagent, polyethylene oxide, is a water-soluble, nonionic polymer having a molecular weight of approximately 5 million. The compound is commercially available from Union Carbide Corp. When the polymer was added to attapulgite suspensions, a thick, stringy mass formed. When this mass was subjected to further treatment, up to 94 percent of the water was released from the system in the form of a clear liquid, leaving a coherent, plastic mass containing the solids.
Polyethylene oxide also flocculated and dewatered suspensions of montmorillonite and plant clay wastes in the same manner but to an even greater extent. More compact masses were obtained with less reagent being used. Based on these observations, attention was focused on the refinement of procedures to be used with polyethylene oxide.
Bench-Scale Batch Flocculation Tests
Test Procedure
The bench-scale tests were conducted by adding a solution of polyethylene oxide from a buret to a beaker containing the clay suspension. The slurry was stirred during the entire flocculation dewatering procedure. Upon addition of the reagent, there was immediate visual evidence of flocculation, but polymer addition was continued until the flocs began to stick together in a coherent mass and clear water began to separate, as shown in figure 1. This degree of solids cohesion must be achieved before dewatering can be continued. At this point, reagent addition was stopped and the separated water was removed by decantation. As the remaining mass was manipulated by stirring, more water separated and was removed. This was continued until no more water could be decanted. The coherent, plastic mass was then pressed between layers of absorbent towels to remove additional water, and the solids content was determined.
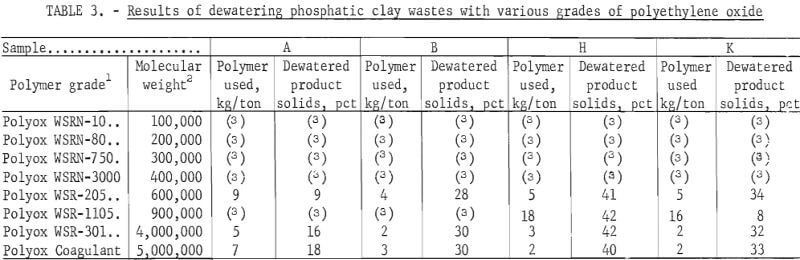
Effectiveness of Polyethylene Oxide Polymers
Eight grades of polyethylene oxide were commercially available. The relative ability of each to flocculate the high-grade clay suspensions and two plant wastes was evaluated (table 3). In each case, the polymer was added as a 0.25-percent aqueous solution; all solutions of the polymer were prepared using distilled or deionized water. The data in the table show that, in general, the dewatering ability of polyethylene oxide is related to its molecular weight, with the Polyox Coagulant grade being superior. For simplicity, the Coagulant-grade polyethylene oxide was termed PEO and is so designated throughout the remainder of the report.
Another interesting relationship is indicated by the amount of polyethylene oxide required for dewatering the different materials. Three of the samples—montmorillonite and the two plant samples—required roughly the same amount of polyethylene oxide, but attapulgite required roughly twice the amount. The possibility that surface area of the suspended solids was the governing factor in the quantity of reagent required was investigated. Surface area of the clay particles dried at 200° C was determined by the BET method, according to procedure followed by Lamont, and preliminary results have supported this idea. The surface area of attapulgite was found to be 140 square meters per gram, and that of montmorillonite was found to be 93 square meters per gram. The surface areas of the 15 plant wastes reported in the characterization study ranged from 27 to 88 square meters per gram.

SEM Examination of Flocculated Systems
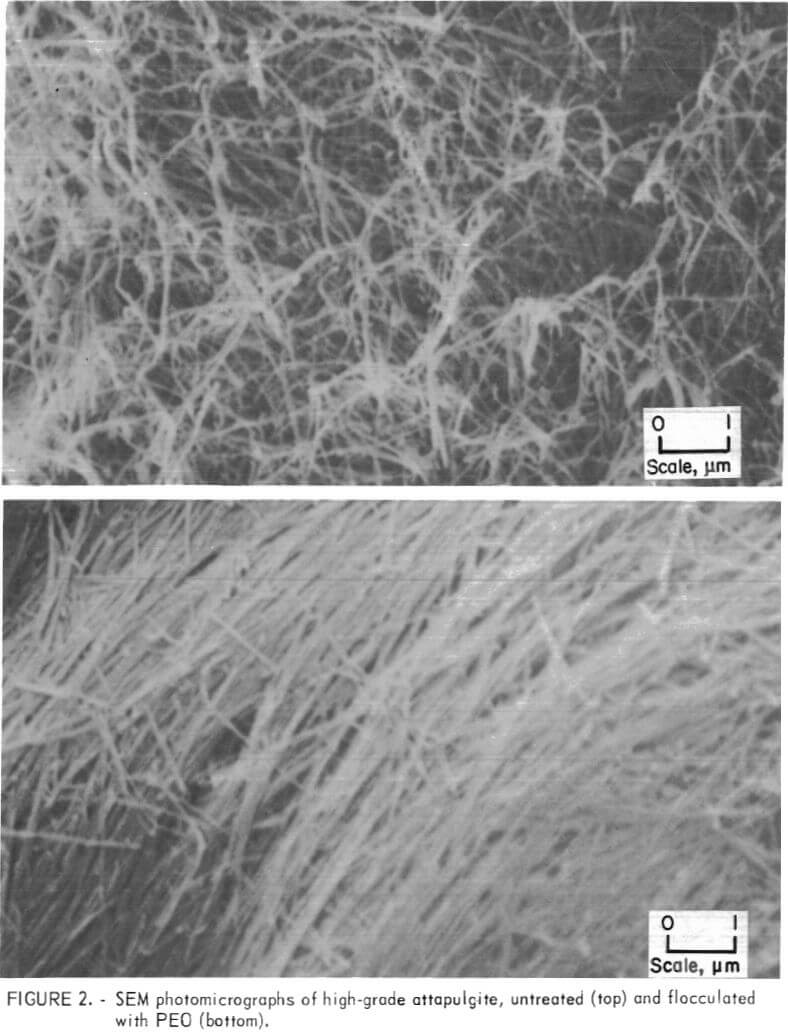
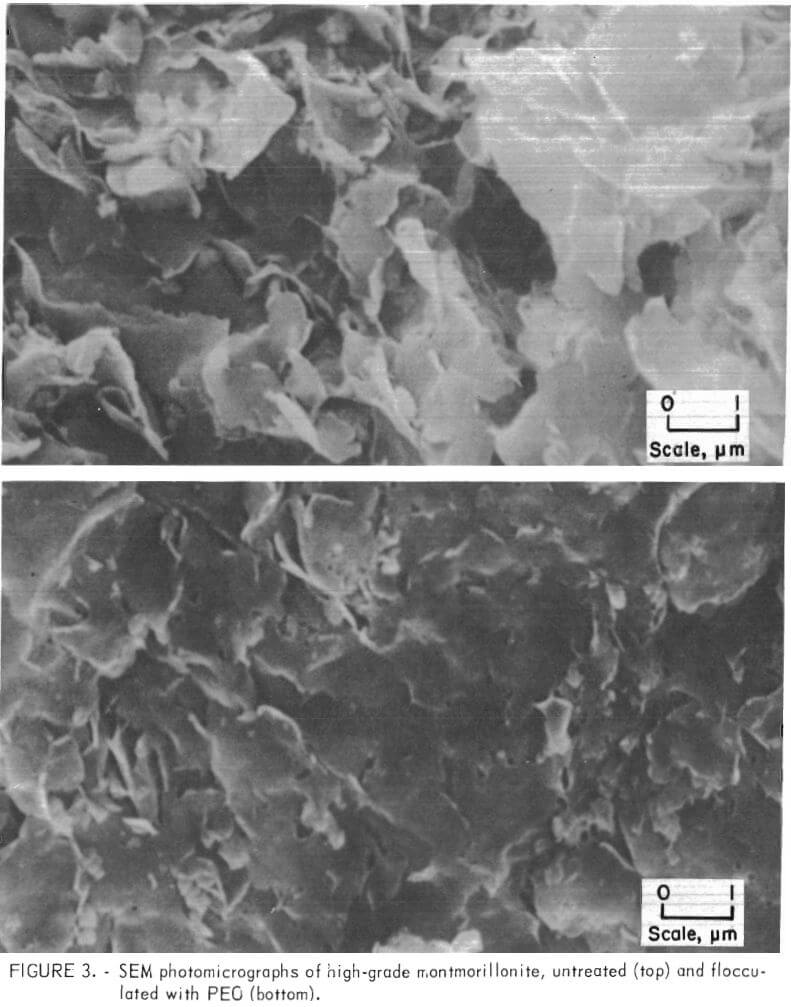
Photomicrographs of high-grade attapulgite and montmorillonite clay systems before and after treatment with PEO are shown in figures 2 and 3. The photographs compare the unflocculated and flocculated clays after critical-point drying. In figure 2, the greatly enhanced alinement of the long, needlelike attapulgite fibers which appeared after treatment probably is due to PEO. This orderly arrangement of the solids in the compacted mass accounts for much of the significant reduction in volume that occurs when the suspended solids have been subjected to treatment with PEO. A similar occurrence was observed for the montmorillonite. Figure 3 shows that the thin flakes of this clay after treatment appear to be flattened and oriented in the same plane.
Tests on Plant Wastes
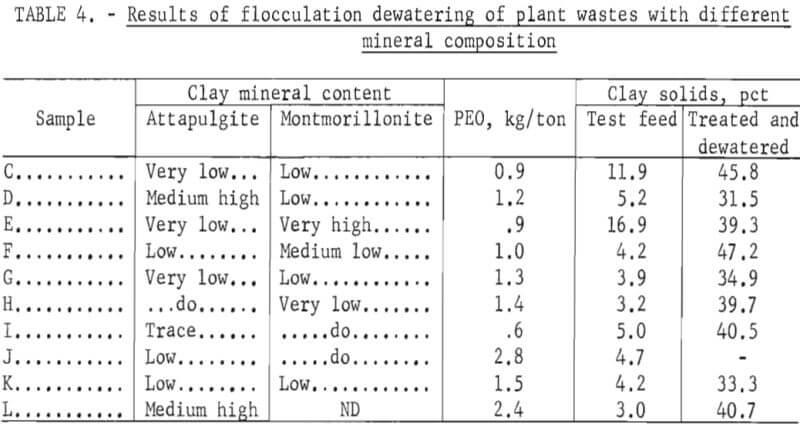
Bench-scale flocculation dewatering tests also were made on a group of phosphate-plant clay wastes. The results of these tests are given in table 4. The data indicate no correlation between PEO requirement and clay composition. To obtain optimum flocculation, each plant-waste suspension had to be evaluated separately.
Effect of Solution Concentration
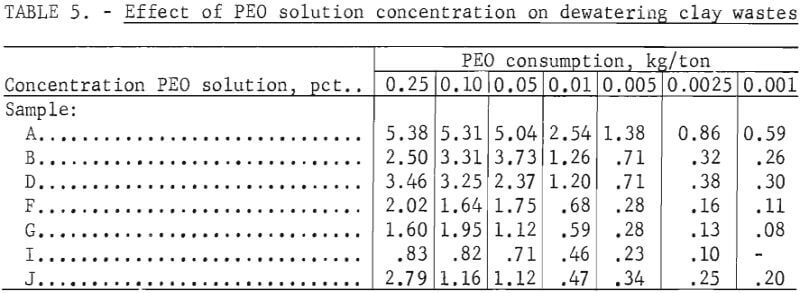
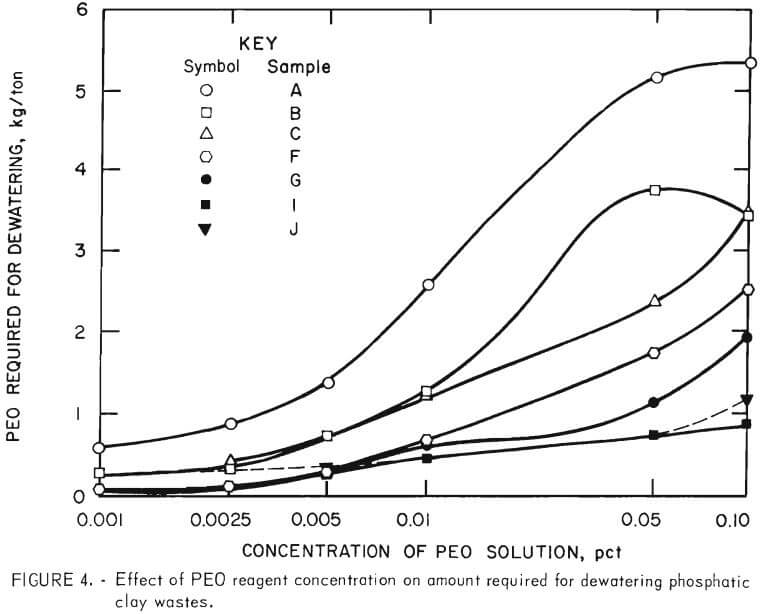
Laboratory tests were conducted to establish the effects of PEO concentration on dewatering the high-grade clay suspensions as well as the five plant wastes. The slurries were treated with PEO solutions of the following percentage concentrations: 0.25, 0.10, 0.05, 0.01, 0.005, 0.0025, and 0.001.
The results of the tests presented in table 5 indicate that, in general, less reagent was required to flocculate and dewater those suspensions with lower PEO concentrations. This effect was more evident at PEO concentrations of less than 0.05 percent, as is shown graphically in figure 4. However, much greater dilution of the reagent was not advantageous. The amount of solution
used was so great with the 0.001-percent solution that the separation of released water from the solid product became very difficult.
Effect of Solids Content in Test Feed
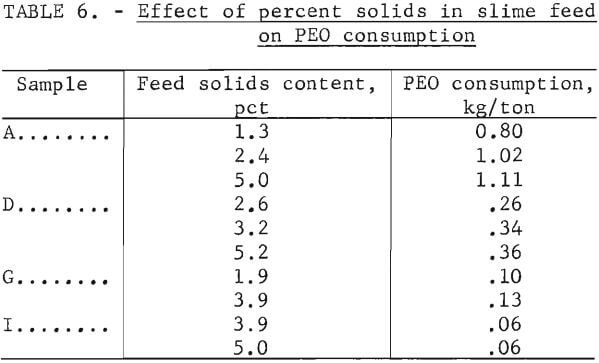
To investigate the effect of varying the solids content on reagent consumption, several of the waste clays were diluted with supernatant water from settled waste clays and then flocculated. The results obtained using a solution of 0.0025 percent PEO are presented in table 6. The data indicate a general trend, showing that more reagent is required with increased percent solids content of feed.
Continuous Flocculation Tests
Preliminary Tests
Tests were initiated to develop a continuous processing method for dewatering phosphatic clay wastes. Several different standard mineral-processing devices were evaluated, including a rotating trommel, a vibrating screen, a screw classifier, and a two-stage dewatering operation consisting of a curved static screen in combination with either a trommel or a vibrating screen.
In the first trial, a plant waste (sample I) of 5 percent solids was combined with the flocculant solution in a launder, mixed additionally by low-pressure tangential flow through a cyclone apparatus, and discharged into a 0.38-meter-diameter by 0.9-meter-long trommel fitted with a 10-mesh wire screen. The flocculated material dewatered very rapidly and collected in consolidated masses of sausage-shaped rolls or balls as it moved through the trommel. The thickened product from the trommel contained 25 to 30 percent solids. The discharge water was clear but contained some flocculated solids that leaked through the first 25 to 30 centimeters of exposed screen. For feed rates of 1.5 to 3.0 liters per minute, PEO consumption was about 1 to 2 kilograms per ton.
In another test, the flocculated waste was effectively dewatered with comparable reagent dosage on a horizontal vibrating screen dressed with a 100-mesh wire cloth. Exposure to the screen vibration caused loss of water and formation of the consolidated thickened solids into small balls appearance. The dewatered product contained 29 percent solids.
A third procedure incorporated the use of a curved, static, wedge-wire screen. In this test, a plant-waste product containing 3.9 percent solids was fed at a rate of 3 liters per minute into a mixing reservoir; PEO was added in the amount of 1.6 kilograms per ton of dry solids. The flocculated material was allowed to overflow onto the screen. Considerable dewatering occurred on the curved screen and a dewatered product of about 17 percent solids was obtained. This product was further dewatered by two methods, (1) to 31 percent solids using a horizontal vibrating screen, and (2) to 27 percent solids with a rotating trommel.
A screw classifier also was successful in continuously dewatering the flocculated waste. Clay waste and PEO were fed into a mixing chamber, mixed with a stirrer, and allowed to overflow into the screw classifier. Clay waste at 3.9 percent solids was fed at 4 liters per minute with a PEO dosage of 2.4 kilograms per ton of solids. The dewatered solids product, which was separated from the water by the screw, contained about 30 percent solids.
In summation, the preliminary tests indicated that, regardless of the method of treatment, the phosphatic clay waste product was dewatered to a range of 25 to 30 percent solids. The minimum quantity of PEO used was about 1.5 kilograms per ton. Attempts to lower the quantity of PEO appreciably resulted in unsuccessful dewatering of the waste products. In these attempts, flocculation occurred, but the flocs were weak, did not adhere to each other, failed to release clear water, and often broke down completely when subjected to the intense agitation of the vibrating screen or when released water was not removed rapidly enough. Hence, testing was continued using the most reliable dewatering device, the trommel. Changes were made in the test method and the apparatus to improve mixing, to reduce loss of solids in underflow, and to facilitate more rapid removal of water from the trommel screen. Improved mixing of the clay waste with reagent was accomplished by using a propeller-type mixer. A 30.5-centimeter section of 48-mesh screen was inserted into the feed end of the trommel to reduce loss of solids in the effluent water. The removal of water from the trommel screen was greatly improved by placing a shallow annular baffle about 0.3 meter from the discharge end.
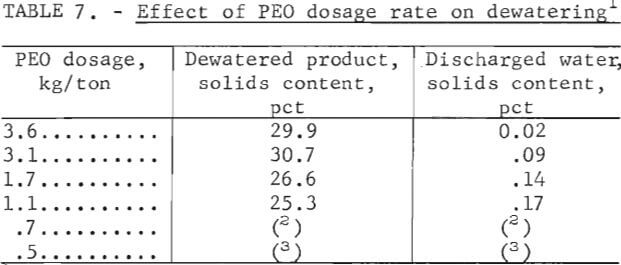
Using this modified system, a sample of phosphatic clay waste containing 4.7 percent solids was treated at a rate of 3.8 liters per minute to investigate the effect of PEO dosage rate on dewatering. The results of this series of tests using a 0.25-percent solution of PEO are summarized in table 7. These data revealed that reducing the PEO dosage also reduced the solids content of the dewatered product and increased the solids content of the effluent water.
Small-Scale Tests
Continuous small-scale tests were made to investigate the following operating variables: Clay waste and reagent mixing, trommel speed, trommel slope, reagent concentration and dosage, feed rate, and retention time.
Description of Equipment
In order to develop data with respect to these various operating conditions, a continuous small-scale 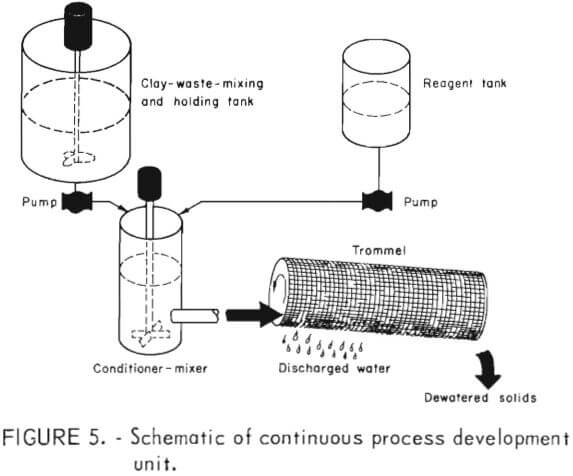 process development unit (PDU) was set up as shown schematically in figure 5. The PDU consisted of a 4,500-liter-capacity, stainless steel tank with provision for continuous mixing of phosphatic clay wastes; a positive displacement pump to feed the waste slurry at predetermined rates; a conditioner-mixer with a propeller-agitator to mix slime and PEO; a 75-liter, stainless steel storage tank with a positive displacement pump to feed the PEO at controlled rates; and a rotary trommel.
process development unit (PDU) was set up as shown schematically in figure 5. The PDU consisted of a 4,500-liter-capacity, stainless steel tank with provision for continuous mixing of phosphatic clay wastes; a positive displacement pump to feed the waste slurry at predetermined rates; a conditioner-mixer with a propeller-agitator to mix slime and PEO; a 75-liter, stainless steel storage tank with a positive displacement pump to feed the PEO at controlled rates; and a rotary trommel.
The conditioner-mixer was a cylindrical vessel with an inside diameter of 26 centimeters and a depth of 35 centimeters. Mixing was performed by a straight-blade propeller mounted on a central shaft driven by a variable-speed motor at the top of the shaft. Feed was introduced into a center well located in the upper part of the conditioner-mixer. Three overflow ports were available for outlet feed but only the lowest port, located 7.6 centimeters, above the bottom was used.
The trommel was fitted with a 10-mesh screen and was 0.34 meter in diameter and 1.8 meters in length. The first 0.5 meter of the trommel was lined with a 48-mesh screen to promote adhesion of the flocculated material. A view of the PDU during operation is shown in figure 6.
Mixing of Clay Waste and Reagent
During the tests, the waste clay and flocculant were fed separately to the center well of the conditioner-mixer as shown in figure 7. The degree of mixing in the conditioner-mixer was critical for obtaining a product that dewatered properly. For example, at a feed rate of 4 liters per minute, discharge from the conditioner-mixer at the 7.6-centimeter overflow level dewatered satisfactorily. When using a higher level overflow port, 14 centimeters for example, the flocculated product disintegrated and passed through the trommel screen with no dewatering. It was assumed that agitation in a deeper column combined with increased retention time resulted in degradation of the flocculated product. As previously mentioned, once dewatering of the flocs occurs, it is essential that released water be removed as rapidly as possible to insure good adhesion of the floes for additional release of water.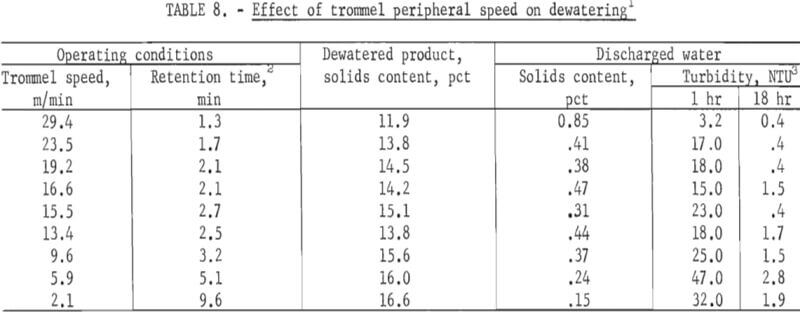
Effect of Trommel Speed
Tests were made using a sample of phosphatic clay waste containing 4.74 percent solids to determine the effect of rotational speed of the trommel on dewatering. The trommel was inclined at 3.2° from the horizontal, and a 0.01- percent PEO solution was fed to the waste-clay at a rate of 0.75 kilogram per ton of dry clay solids. The slime feed rate was 3.8 liters per minute. Peripheral speed of the trommel ranged from 2.1 to 29.4 meters per minute.
The residence time in the mixer and trommel also was established for each test. Samples were taken of the flocculated material discharged from the conditioner-mixer, the dewatered product, and the discharged water. The solids content of each sample was determined. In addition, the turbidity after 1 hour and 18 hours was determined on the discharged water samples. The results of these tests are shown in table 8. The data show that, in general, as the peripheral speed increased, the solids content of the dewatered product decreased. At the slower speeds, the retention time increased, resulting in a better dewatered product.

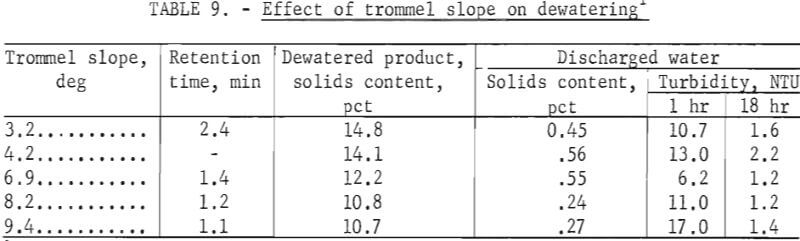
Effect of Trommel Slope
Tests were made to determine the effect of the trommel’s angle of inclination on dewatering. The results of these tests are given in table 9. The data indicate that increasing the trommel’s slope decreased both the solids content of the dewatered product from 14.8 to 10.7 percent and the retention time from 2.4 to 1.1 minutes. In addition, percent solids of the effluent water decreased from 0.55 to 0.24 percent as the angle was increased from 6.9° to 8.2°.
Effect of Reagent Concentration and Dosage
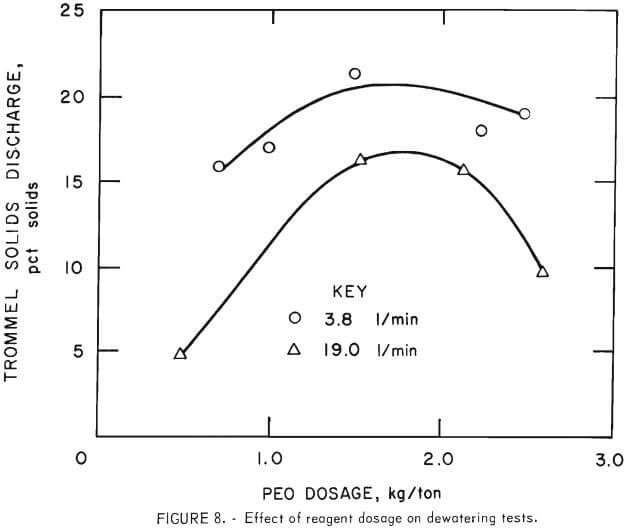
The effects of reagent concentration and dosage were determined to be significant. The results of continuous tests using PEO solutions of different concentrations correlated well with the results of previously reported, laboratory batch tests. In both series of tests, as the dilution of reagent increased, the amount of PEO required for dewatering decreased; however, the solids content of the dewatered product also decreased. Although emphasis was placed on establishing conditions to minimize PEO dosage, it became evident in continuous testing that there was an optimum quantity of reagent necessary to produce maximum solids content. Additions of reagent above this optimum of 1.5 to 2.0 kilograms per ton yielded a product of lower solids content. Results showing this behavior are presented in figure 8.
Effect of Clay-Waste Feed Rate
Tests were made to determine the capacity of the trommel by increasing the treated-clay-waste feed rate. Successful dewatering of the clay waste was obtained at feed rates of 3.9 to 39.0 liters per minute. The dewatered products from all of these tests were similar and had a solids content that ranged from 15 to 17 percent. The effluent waters from these tests contained from 0.10 to 0.74 percent solids, which settled rapidly. After 1 hour, the effluent contained less than 1 part per million of suspended solids.
Retention Time
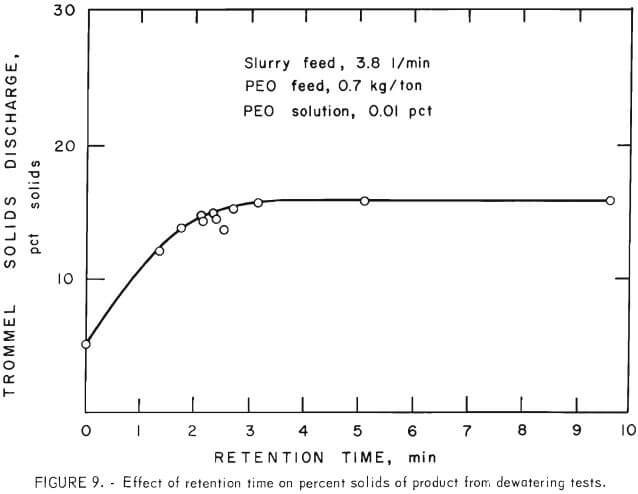
Trommel speed, trommel slope, and feed rate affected the percent solids of the dewatered product because they affected retention time. The curve in figure 9 shows that the solids content of the dewatered product increased rapidly with increased retention time up to 3 minutes. However, the percent solids of product did not change significantly with retention time greater than 3 minutes. This same trend in the percent solids of product was observed in other tests where changes in retention time resulted from variations in trommel rotation speed and slope.
Large-Scale Tests
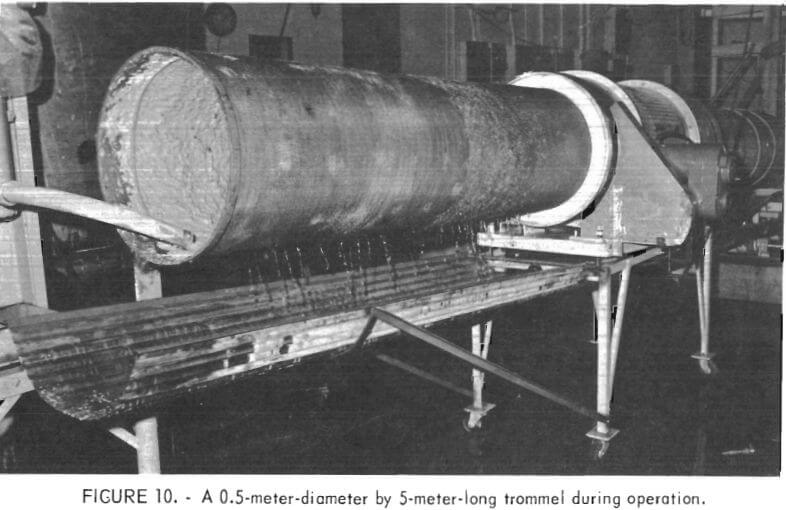
A larger trommel was constructed to determine whether satisfactory dewatering of the clay wastes would be obtained if feed rates and equipment size were increased. This trommel was 0.5 meter in diameter and 5 meters long and fitted with 10-mesh screen. One meter at the feed end of the trommel was lined with a 48-mesh screen. During operation, the peripheral speed of the trommel was set at 12 meters per minute, which had been found satisfactory for dewatering operation with the smaller trommel. The larger trommel was incorporated into the same PDU circuit as shown in figure 5. With the exception of the larger trommel, all of the equipment used for the continuous small-scale tests was also used for the continuous large-scale tests. An overall view of the trommel in operation is shown in figure 10.
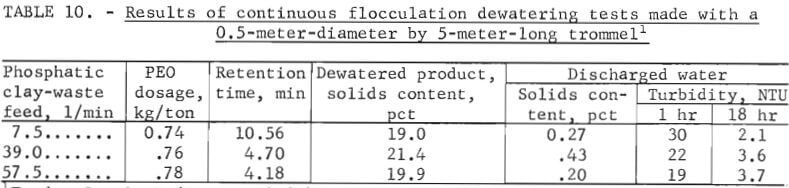
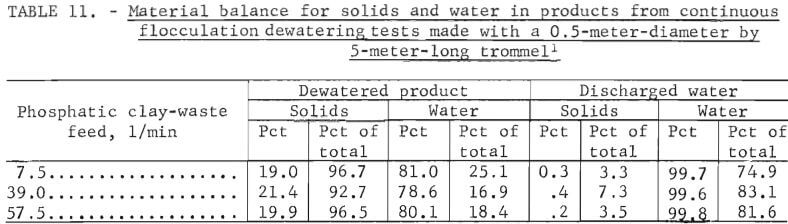
The results of initial tests are presented in table 10. These data show that, in general, performance of the larger trommel performed better than the smaller unit. At feed rates of 7.5 to 51.5 liters of clay wastes per minute, the unit yielded dewatered products that contained from 19.0 to 21.4 percent solids. The solids content was relatively consistent considering the broad range of feed rates used in the tests. A material balance showing distribution of solids and water in trommel products is shown in table 11. These data show that 92.7 to 96.7 percent of the solids was recovered in the dewatered product, and 74.9 to 83.1 percent of the water was removed as discharged water.
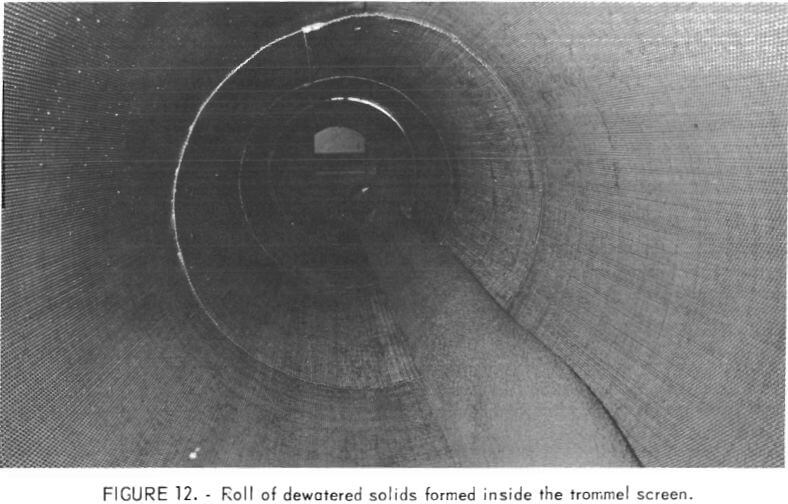
Closeup views of the feed end, the inside, and the discharge end of the trommel during operation are shown in figures 11, 12, and 13, respectively. The view at the feed end shows clearly how the solids in the flocculated mixture immediately begin to coalesce and consolidate while large quantities of effluent water are removed simultaneously. The initial forming of the solids into a roll inside the trommel also is evident at the feed end of the unit. The roll along the inside length of the trommel is shown in figure 12. In figure 13 the dewatered product is discharging from the trommel and the flow of effluent water removed during the dewatering also is shown. The texture and physical character of the discharged product is indicated by the
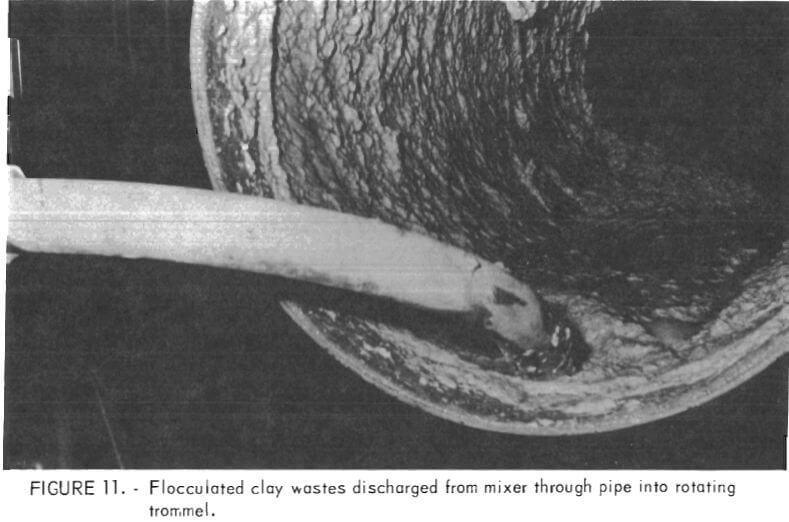

manner in which the consolidated mass breaks away to fall into the receiving hopper. This physical character is in sharp contrast to the gelatinous, colloidal suspension of phosphatic clay waste going in as feed to the dewatering system.
Summary and Conclusions
In this study, a large number of reagents including organic and inorganic compounds were tested for utility in flocculating and dewatering of phosphatic clay wastes. Several polyethylene oxide polymers were identified as reagents that functioned satisfactorily in a specialized flocculation dewatering method that resulted from the research. The flocculation dewatering system was effective on clay-waste samples of different mineral compositions that were obtained from plants and deposits in the central Florida phosphate region.
Successful dewatering tests were made in bench-scale batch tests and in continuous tests with a trommel as large as 0.5 meter in diameter by 5 meters long and at clay-waste feed rates as high as 57.5 liters per minute. In the larger trommel tests, a sample of plant waste containing about 4 percent solids was dewatered to about 20 percent solids; about 0.8 kilogram of PEO per ton of dry waste clay solids was required. The retention or processing time for the solids in the treatment system was about 4 minutes.
The primary requirements for the continuous treatment system are (1) to properly mix the clay-waste feed with the PEO reagent; (2) transfer the mixed solids and reagent to equipment such as a trommel that will allow rapid removal of water as it is released in processing; and (3) to provide a mechanical means for physically manipulating the mass of solids to promote cohesion of the solids and additional release of effluent water.
Important variables of the process are (1) characteristics of mixing used for the feed and the reagent, (2) the amount of PEO used for flocculation, (3) concentration of the flocculating reagent solution, (4) character of the physical manipulation used to promote water removal, and (5) solids cohesion and retention time for the material during processing.
Large-scale PDU tests will be continued to develop engineering data for proper design of a field test unit (FTU). The FTU will have a feed capacity of about 400 liters per minute. Upon completion of the engineering design, construction of the unit will be expedited. The unit will be set up at various operating plants in Florida to develop operating data more suitable for scaleup to full size operations and for evaluating the economics of the process.
The Bureau of Mines, U.S. Department of the Interior, as part of its mission to effect pollution abatement, conducted research to devise means for disposing of phosphatic clay wastes and reclaiming mined land. Part of the investigation was performed in a cooperative research project with 10 phosphate companies in Florida. The colloidal properties of the phosphatic clay suspensions, called slimes, make them difficult to dewater and dispose of without impoundment storage above ground. Various reagents to flocculate and dewater these plant wastes were evaluated, and a polyethylene oxide polymer was found to function in a specialized flocculation dewatering technique. The flocculation dewatering method was investigated in bench-scale batch tests and applied in continuous dewatering tests using standard mineral-processing equipment. In continuous tests at feed rates of up to 58 liters per minute, wastes at 4 percent solids were dewatered to about 20 percent solids. About 0.8 kilogram of polymer was used for each metric ton of dry waste clay solids; processing time was about 4 minutes. Requirements for the treatment system are (1) proper mixing of the clay-waste feed and flocculant, (2) rapid removal of water as it is released, and (3) physical manipulation to promote cohesion of solids.
